Many industrial chemical processes and tests require chemical indicators to determine when certain thresholds are reached. Which processes a chemical indicator is used varies depending on the intended application.
Chemical indicators are also used to determine the presence of certain chemicals, such as specific pollutants in water. Although it is not as accurate or as precise as spectroscopy or chromatography in analysing chemical content, it is a simpler and more convenient method.
Using chemical indicators in laboratory experiments like titration is relatively easy. In fact, it is one of the basic experiments conducted by secondary school chemistry students. It does not require expensive laboratory equipment like chromatographs.
In this post:
What is a Chemical Indicator?

A chemical indicator relies mainly on colour change to show the presence of certain chemicals, similar to litmus paper. The change in colour is an indicator that a threshold concentration of a certain type of chemical is reached.
Examples of how chemical indicators are used include:
- Alkaline-acid solutions: chemical indicators are very useful for determining the presence of acidic or alkaline substances in a solution at certain levels of concentration. The levels of sensitivity of chemical indicators vary.
- Methyl yellow: one commonly used chemical indicator is methyl yellow. An alkaline solution becomes yellow in colour when methyl yellow is added. If you gradually add acid in an alkaline solution with methyl yellow, the solution will remain yellow until it is neutralised. It will suddenly change into red when the pH level of the solution becomes 7 or neutral.
- Low concentration needed: most chemical indicators are visible even at a very low concentration. For example, methyl yellow is visible even at a concentration of a few parts per million parts of solution. At very low concentrations, indicators don’t have any significant chemical influence on the solution. Chemical indicators are used to determine the end point of titrations.
Based on the chemical reactants involved, chemical indicators are classified into three categories:
Chemical indicators have specific ranges when it comes to detecting acids, bases, or specific chemicals. Although most indicators change in colour, some change in turbidity (transparency) as a sign of neutralisation or presence of certain chemicals.
For example, a trace amount of iodine is used as an indicator in chemical reactions between a solution of cyanide and silver salt. The solution maintains its clarity until a critical level of soluble silver cyanide is formed.
The solution becomes turbid when more silver salt is added as insoluble iodide is formed because of the excess silver ions. In this case, the chemical indicator actually participates in the chemical reaction.
How Do Chemical Indicators Work?
Chemical indicators may or may not participate in the chemical reactions that are intended to be detected. Some indicators change colour or form precipitates when their ions dissociate.
Even if chemical indicators do participate in a chemical reaction, their concentration is such a negligible amount that they tend not to have any significant effect on the main reaction. Some chemical indicators have very narrow and sensitive range.

For instance, one example of a sensitive oxidation-reduction indicator is ferrous 1,10-phenanthroline.
The colour of the indicator changes from red to pale blue if the oxidation potential is increased to 1.08 volts from 1.04 volts. This is a very sensitive chemical indicator that does not participate significantly in the oxidation-reduction reaction in a solution.
Another common example of a very sensitive chemical indicator with a narrow range is diphenylcarbazone. It indicates an increase in mercuric ion concentration from 0.000001 to 0.00001 mole per litre. The colour of the indicator changes from yellow to violet.
Indicators of chemical change
Depending on the type of chemical indicator and the chemical reaction involved, there are several ways you can observe chemical change. Some of the most common observable and measurable indications are:
- Change in colour
- Formation or disappearance of precipitate
- Bubble or gas generation (can also include a change in smell)
- Change in taste if the compound is edible (example: sour milk)
- Change in temperature (either exothermic or endothermic)
- Release of photons (luminescence)
- Change in volume or density
- Combustion or explosion
However, not all of these signs can be conclusive indicators of chemical change. A chemical change is the same as the change in the molecular composition of substances or reactants.
For example, if you mix equal portions of blue paint with yellow paint, the result would be green. There is change in colour but chemically, the two substances did not change but merely mixed homogeneously.
On the other hand, a seeming absence of any indications or changes in substances or reactants does not necessarily mean absence of chemical change. For instance, when sunlight strikes the skin, cholesterol molecules undergo changes that result in the formation of vitamin D – we can’t see that change.
Virtually all biochemical reactions are hard to detect even in laboratory setups like in Petri dishes. Enzyme reactions cannot be directly observed.
For example, when you eat bread, the salivary amylase breaks down the starch in the bread into maltose but this is not observable by the naked eye. It is only when you use sophisticated laboratory instruments and methods that you can detect the subtle chemical changes.
What Does a Subscript Indicate in a Chemical Formula?
The subscripts in a chemical formula indicate the number of atoms in a molecule. A formula is written as the letter symbol of the elements in a compound with number subscripts.
If there is no subscript, it is simply assumed to be one. For example, the chemical formula for water is H20. This means that a molecule of water has two atoms of hydrogen and one atom of oxygen.
Chemicals, which could either be elements or compounds, can be represented or written in several ways. Some ways convey more information than others. The most common way is by its common name such as “table salt”. The same chemical, table salt, can also be called by its chemical name, which is sodium chloride.
Other more formal ways is by using chemical formulas:
- Empirical formula – shows the elements that composed a compound, like NaCl (which is the same as table salt and sodium chloride)
- Molecular formula – this is more detailed than an empirical formula because it tells you something about the number of atoms per molecule of a compound
- Structural formula – as the name suggests, this type of formula indicates the way the atoms in a molecule are arranged
To illustrate the differences between these formulas, let’s use benzene as an example. Benzene is an aromatic hydrocarbon:
Empirical: CH
Molecular: C6H6
Structural:
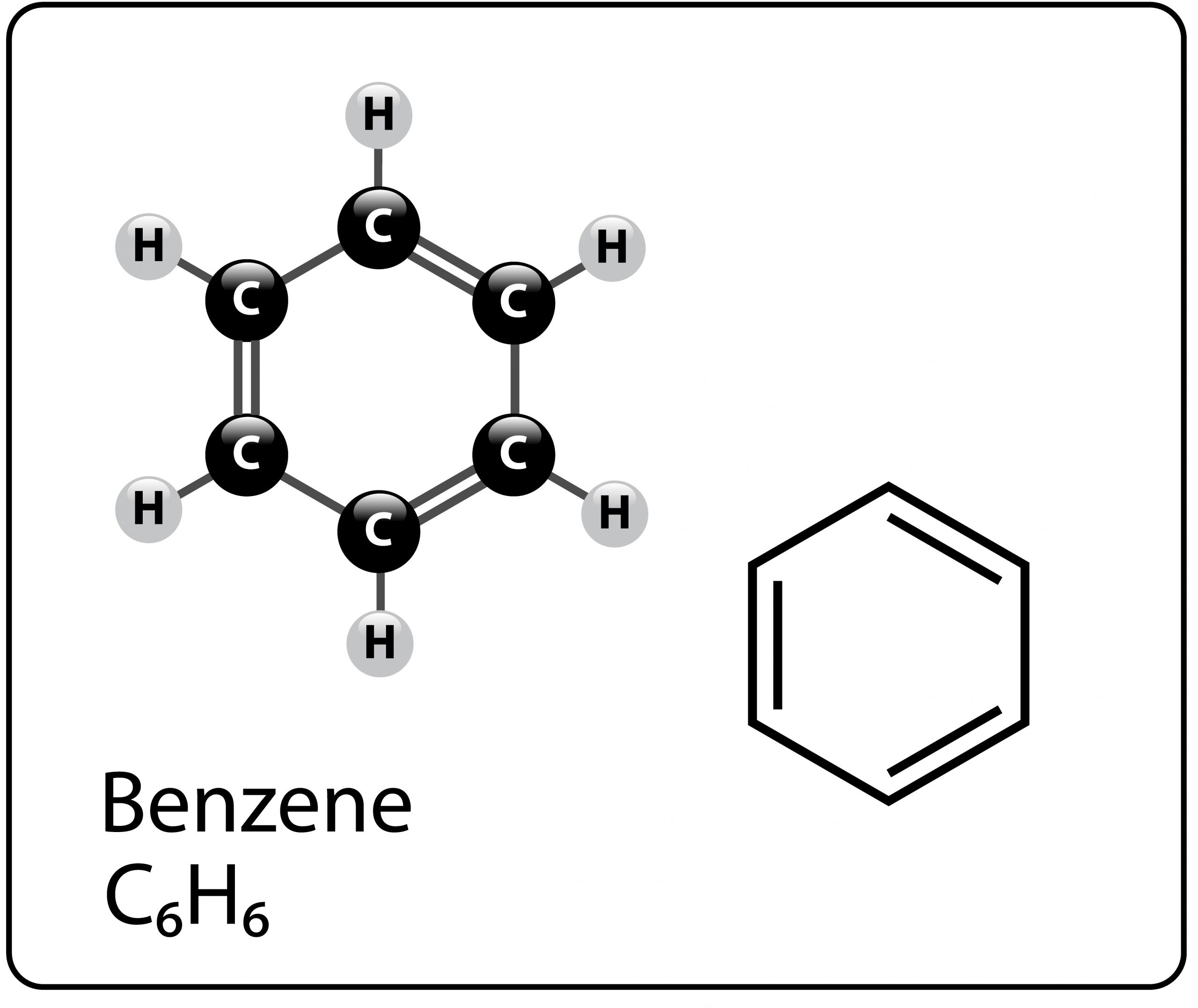
As you can see, the structural formula can be written in different ways. Some more complicated chemicals are also illustrated as three-dimensional graphics.
Chemical indicators for sterilisation
Depending on the type of establishment or institution, the sterilisation process can be simple or complicated, requiring protocols and specific parameters.
Food processing factories, pharmaceutical factories, biological laboratories, and hospitals are some examples of establishments that require strict sterilisation protocols.
Chemical indicators are needed to determine whether certain standards or parameters of sterilisation are achieved. Chemical indicators for the sterilisation process are usually standardised in the form of testing kits (strips, labels, card, pellets, and tapes).
The chemicals in a testing kit may undergo a physical or chemical change that is easy to identify, like change in colour after exposure under specific parameters like time, temperature, and sterilising agent.
Chemical indicators for sterilisation are grouped into six classes:
- Class 1: process indicators, which differentiate between processed and unprocessed items
- Class 2: for specific tests based on standards like the Bowie Dick test that determines the efficiency of air removal and steam penetration in a chamber
- Class 3: indicators under this category react to a single variable such as temperature
- Class 4: these indicators react to two or more critical parameters like exposure to heat for a specific period of time
- Class 5: these indicators react to a wide range of parameters over a range of sterilisation cycles. They are commonly used for pack monitoring
- Class 6: these are emulators or cycle specific indicators. For instance, strips that can monitor pre-vacuum steps within a few minutes
Chemical Indicators for Steam Sterilisation
Chemical indicators for steam sterilisation are classified either Class 2, like the Bowie Dick test, which determines steam penetration in a chamber, or Class 6 that verifies that a sterilisation cycle like steam sterilisation stage has been done.
Chemical indicator for lipids
Lipids are among the fundamental biochemical substances found in cells and form the protective cell membranes. Lipids or lipoproteins are also found in the blood and carry cholesterol. These lipoproteins are classified as:
- High-density lipoprotein (HDL), or “good” cholesterol
- Low-density lipoprotein (LDL), or “bad” cholesterol
Lipid content of a mixture can be tested using Sudan III indicators. These are coloured, fat-loving molecules. If a dyed layer is formed in water, lipids are present, but if the colour is evenly distributed, no lipid is present.











