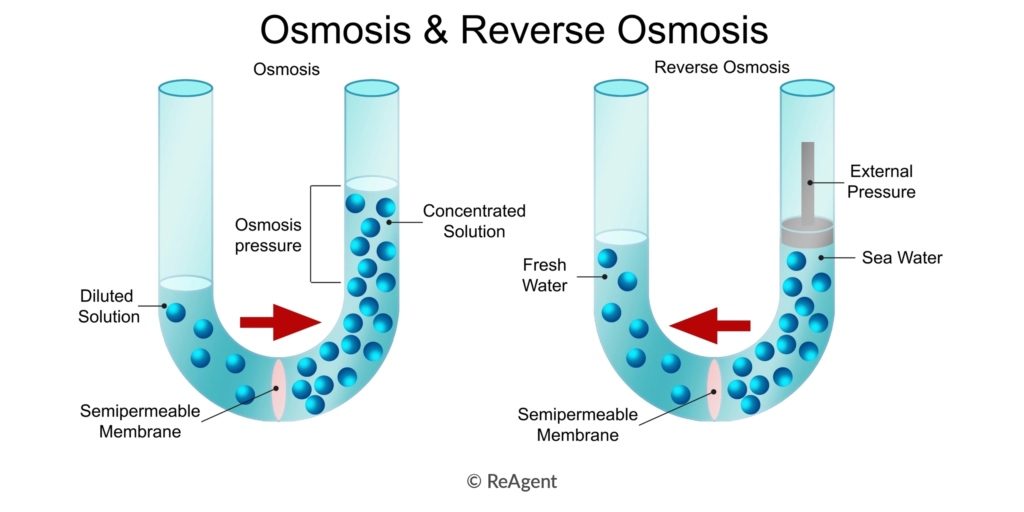
Osmosis works through the natural movement of water through semi-permeable membranes, evening out the saturation of dissolved particles between two bodies of water.
Reverse osmosis, meanwhile, relies on pressure to push the water through, moving it from a high concentrate into a lower concentration solution.
Both processes rely on semi-permeable membranes, but their mechanisms, applications, and energy requirements differ when it comes to the fundamentals of the processes. Osmosis is a natural process that occurs, while reverse osmosis is a man-made principle.
Both of these are also vital to the production of water in different forms, from drinkable to ultrapure. Understanding these processes not only clarifies fundamental biological concepts but also reveals the technologies behind producing clean water, including demineralised water and reverse osmosis systems in industrial settings.
In this post:
Key Takeaways
Water naturally moves from areas with fewer dissolved substances to areas with more in osmosis
Reverse osmosis uses pressure to push water through a membrane, removing impurities
These processes are common in desalination, industrial water treatment, and producing drinking water
Both processes matter, but reverse osmosis is key for purified water in commercial and industrial settings
What Is Osmosis?
As we mentioned above, osmosis is a natural process. During osmosis, water molecules move across a semi-permeable membrane from a lower solute concentration to a higher one.
This process, once started, does not stop until equilibrium in concentrations are reached, meaning the solute is balanced across both sides of the membrane.
Osmosis does not require any energy input, making it a passive form of transport that is vital for all living organisms. This in turn makes it valuable for everything from biological existence to industrial processes.
Companies involved in chemical and water processing often leverage these natural principles when developing solutions that require controlled water movement or solute separation.
How Does Osmosis Work?
Osmosis is driven by osmotic pressure, which is the force exerted by a solvent when it moves through a semi-permeable membrane to equalise solute concentrations.
This principle is evident in many simple experiments, such as placing a raisin in water. Over time, the raisin swells as water moves into it through the skin, demonstrating how water naturally travels to balance concentrations.
This happens because plant cells use osmotic pressure to maintain hydration and structural integrity, making sure that the plant can grow properly. Without this function, plant life would likely not exist as we know it, as would all biological matter.
In industrial applications, understanding osmotic pressure is essential when designing systems for water treatment or chemical processing. Here, the processes use artificial membranes, but the principle is the same.
Everyday Examples of Osmosis
Osmosis occurs in a wide range of daily and natural processes. In plants, roots rely on osmosis to absorb water from soil, which supports growth and the transport of nutrients to leaves and stems. In humans, osmosis in the kidneys ensures proper hydration, reabsorbing water from filtrate and maintaining electrolyte balance.
Food preservation is another area where osmosis is applied. Salting or sugaring foods draws moisture out of the product, which reduces bacterial growth and prolongs shelf life. This principle has been utilised for centuries in pickling, curing meats, and making preserves.
Even in industrial or laboratory settings, osmosis can be observed when working with solutions and chemical storage. This is especially true for contract chemical manufacturing, where understanding the movement of water through membranes allows chemical bottling companies to manage solutions more effectively than through other methods, helping maintain stability and quality during storage and transport thanks to the natural equilibrium achieved.

What Is Reverse Osmosis?
Unlike the natural process of osmosis, reverse osmosis is a manufactured system. It works by using pressure to push water through the same semi-permeable membranes, making water move from high solute concentration to a low solute in order to remove impurities. This process requires energy input in the form of applied pressure to overcome natural osmotic pressure.
The main reason for using reverse osmosis is to take out dissolved salts, bacteria, viruses, and chemicals from water. This makes it a key technology for drinking water purification, desalination of seawater, and industrial water treatment.
Reverse osmosis is widely adopted because it can produce water that meets stringent quality standards required for consumption, laboratory use, and chemical manufacturing.
Reverse osmosis is also the foundation for producing demineralised water, which is used extensively in laboratories and industries that require water with very low mineral content. This process ensures that water does not interfere with chemical reactions or industrial systems, highlighting its importance in modern technology.
How Reverse Osmosis Systems Work
Reverse osmosis systems typically operate in several stages. The first stage is pre-filtration, which removes larger particles such as sediment and debris that could damage the membrane.
Once done, the water is then pushed through a semi-permeable membrane under pressure, which removes dissolved salts, microorganisms, and chemical impurities. Finally, post-filtration improves taste and removes any remaining impurities to ensure water quality.
Reverse osmosis systems are scalable and can be found in residential, commercial, and industrial environments.
In industry, they support processes that require ultrapure water, such as pharmaceuticals, electronics manufacturing, and contract chemical production. The reliability of reverse osmosis makes it a critical component for applications where water quality is non-negotiable.
Understanding what reverse osmosis is also helps companies select and maintain equipment properly. Many reverse osmosis providers publish guides and case studies to illustrate how membrane performance, pressure, and flow rates affect purification efficiency.
Key Differences Between Osmosis and Reverse Osmosis
While both processes involve the movement of water through a semi-permeable membrane, their mechanisms, energy requirements, and applications are fundamentally different:
Direction of Water Flow:
- Osmosis: Water moves naturally from low to high solute concentration.
- Reverse Osmosis: Water is moved from high to low solute concentration using pressure.
Energy Requirement:
- Osmosis: Low to none, as it naturally occurs when there is imbalance between concentrations
- Reverse Osmosis: Requires applied pressure to overcome natural osmotic flow
Process Type:
- Osmosis: Natural and occurs spontaneously in living organisms and ecosystems
- Reverse Osmosis: Artificial, engineered for water purification and industrial applications
Primary Applications:
- Osmosis: Supports biological functions such as plant water uptake and kidney function in humans
- Reverse Osmosis: Used for water purification, desalination, industrial water treatment, and producing demineralised water

Examples of Osmosis in Biology and Nature
Osmosis is fundamental to life. In plant cells, it allows roots to absorb water from soil, supporting growth and nutrient distribution. The maintenance of turgor pressure ensures that plants remain upright and resilient against environmental stress.
In humans, osmosis is central to kidney function. Water is reabsorbed from filtrate to maintain hydration and electrolyte balance, preventing dehydration and supporting homeostasis. Cells also rely on osmosis to regulate internal water content, ensuring proper metabolic function.
In addition to organisms, osmosis affects natural ecosystems. For example, the movement of water in soil affects the availability of nutrients, influencing plant growth. Understanding these principles is crucial for agriculture, environmental management, and the design of industrial water treatment systems.
Applications of Reverse Osmosis in Water Treatment
One of the largest uses of reverse osmosis is in producing potable water, especially from seawater and brackish water supplies. This makes it vital for regions with limited freshwater resources. Desalination plants use reverse osmosis to provide safe drinking water to millions of people worldwide.
Industrially, reverse osmosis ensures that water used in laboratories, pharmaceuticals, and chemical production meets exacting standards. This includes producing demineralised water for applications where even trace minerals could interfere with chemical reactions or machinery.
In municipal water systems, reverse osmosis improves water quality by removing contaminants that conventional filtration cannot eliminate. This has significant public health benefits, ensuring access to clean and safe drinking water.
Companies involved in water treatment and chemical bottling rely on reverse osmosis to deliver consistent and high-quality water for multiple industrial applications.
Which Process Is More Useful for Water Purification?
Reverse osmosis is more practical for water purification due to its ability to remove a wide range of impurities, including salts, bacteria, viruses, and chemical contaminants.
Osmosis, while critical for biological systems, cannot produce purified water because it moves water according to natural concentration gradients.
Industries and municipalities use reverse osmosis to supply drinking water, desalinate seawater, and produce water for industrial processes. Its reliability and efficiency make it the method of choice when high water quality is required.
The scalability of reverse osmosis systems allows them to serve households, large industrial plants, and entire cities. Integrating reverse osmosis with additional chemical and filtration processes ensures that water meets specific purity standards for any application.
Conclusion
Osmosis and reverse osmosis are both essential for different purposes. Osmosis is a passive, natural process that sustains life by regulating water in cells and ecosystems. Reverse osmosis is an active, energy-dependent process designed to produce clean water for consumption, industrial use, and chemical applications.