Acid-base reactions are known as neutralisation reactions. They’re characterised by the formation of salts and water, which typically have neutral pH levels.
The final products of a neutralisation reaction between an acid and a base may have a non-neutral pH level if the reactants aren’t balanced. If there’s excess acid, the solution will be acidic. Conversely, if there’s excess base, the solution will be basic.
Acid-base reactions are exothermic because bonds are broken and heat energy is released. Neutralisation is an example of a double displacement reaction, as the ions of the acids and bases exchange places during the reaction.
Many industrial processes, including chemical manufacturing, require neutralisation reactions. A variety of new products can be synthesised in this manner.
Neutralisation reactions are also often used to treat pollution as they can neutralise the acidic effluents that may accidentally be released into nature. This type of reaction also has several applications in agriculture, such as in the treatment of acidic soil.
In this post:
What Happens When There is an Acid and Base Reaction?
An acid-base, or neutralisation, reaction is a common type of chemical reaction that occurs in nature.
For example, the pH level of human blood is slightly basic at pH 7.40. Our blood pH may fluctuate slightly, depending on our physiological conditions and physical activity. Carbonic acid (H2CO3), bicarbonate ion (HCO3–) and carbon dioxide (CO2) serve as buffers to maintain the right balance, as shown in the illustration below.  As you can see, the reactions are in dynamic equilibrium. This means the reactions are happening forwards and backwards simultaneously. Bicarbonate ions combine with excess hydrogen ions to form carbonic acid, which in turn decomposes into water and carbon dioxide, and vice-versa.
As you can see, the reactions are in dynamic equilibrium. This means the reactions are happening forwards and backwards simultaneously. Bicarbonate ions combine with excess hydrogen ions to form carbonic acid, which in turn decomposes into water and carbon dioxide, and vice-versa.
When an acid reacts with a base, they undergo neutralisation and their opposite pH levels cancel each other out. This happens through the double displacement of ions or, put simply, the ions switch partners. In a balanced or complete chemical reaction between a base and acid, the resulting solution (product) has a pH level of 7. 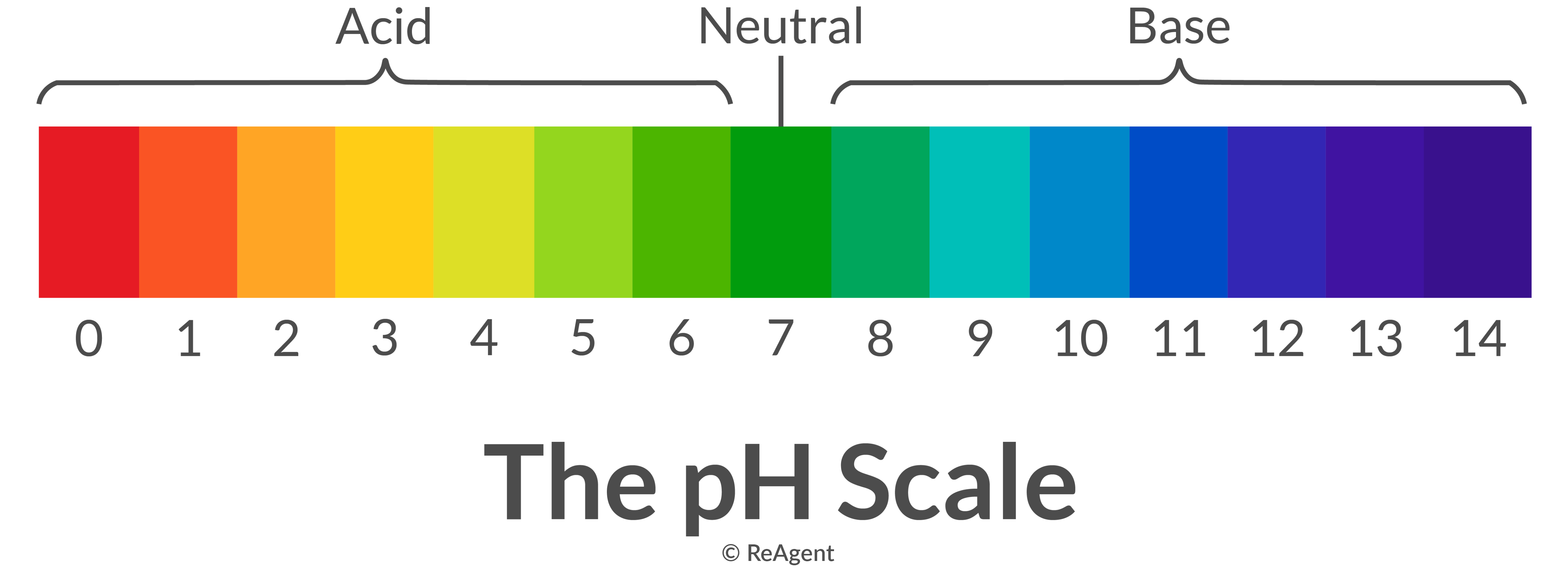
There are three theories that explain how reactions between acids and bases occur:
- The Arrhenius theory – this is based on the observation that both acids and bases dissociate in water into ions. According to this theory, acids produce hydrogen ions and bases produce hydroxide ions. When these ions meet they form water, while their respective other constituent ions form salt.
- The Brönsted-Lowry theory – this definition states that acids are proton donors and bases are proton acceptors. This means that during an acid-base reaction, the protons of an acid, which are in the form of hydrogen ions, are attracted by the base.
- The Lewis theory – according to the Lewis theory, an acid is an electron-pair acceptor while a base is an electron-pair donor. During an acid-base reaction, an electron-pair is transferred from the base to the acid. This definition is much more flexible compared to the Brönsted-Lowry theory.
How to Distinguish Between Acids and Bases
You can normally distinguish acids and bases by their pH levels, which can be accurately and precisely measured using a pH meter. An acid has a pH level lower than 7 and a base has a pH that’s greater than 7.
The pH range goes from 0 to 14. It’s a logarithmic scale that’s expressed in terms of hydrogen ion concentrations in per litre equivalents of aqueous solutions. The formula for this scale is shown below.
pH = −log[H+]
You can also use indicators like litmus paper to determine the acidity or basicity of a solution. However, using indicators is neither precise nor accurate. It’s not quantitatively expressed but merely qualitatively determined. 
Three examples of acids and bases
Acids and bases can be organic or inorganic and either strong or weak. They can also be classified according to the three theories outlined above. Here are some examples of acids and bases.
Nitric acid
Nitric acid has the formula HNO3. It’s classified as a strong acid based on its dissociation constant. Nitric acid is mainly used in the manufacture of fertilisers.
Lactic acid
Lactic acid (C3H6O3) is an organic acid. It’s a metabolic by-product of organisms like bacteria and humans. When oxygen levels are low, the body breaks down carbohydrates and the muscles produce lactic acid as a waste product. Lactic acid is responsible for the muscle pain you might feel after a heavy workout.
Sulphuric acid
Sulphuric acid is another strong inorganic acid. It’s virtually ubiquitous in industry and is used to manufacture everything from pharmaceutical products and detergents to fertilisers and inorganic salts. Sulphuric acid is crucial in refining petroleum products and is commonly used as an electrolyte for lead acid batteries. It’s also used as a reagent in manufacturing plastics.
Lithium hydroxide
Lithium hydroxide has the chemical formula LiOH. It’s a strong base that’s commonly used as an electrolyte for lithium batteries and as a cleaning agent for fabrics.
Calcium hydroxide
Calcium hydroxide (Ca(OH)2) is mainly used in sewage treatment, paper manufacturing, and food processing.
Diethynylbenzene dianion
Diethynylbenzene dianion (C6H4C2-4) is an organic base that’s considered the strongest known base as it has such a high affinity for protons. It’s actually an ion and a substituent in a benzene ring. Diethynylbenzene dianion is mainly used in laboratories to deprotonate weak acids.
How to Calculate the pH of an Acid and Base Mixture
The pH of the resultant solutions from the reactions of weak acids with strong bases (or vice versa) is not necessarily neutral. The final pH will depend on the concentration and strength of the base or acid.
By definition, pH is the concentration of hydronium ions in an aqueous solution. This can be mathematically expressed as:
pH = −log 10[H3O+]
To calculate the pH of a solution after a weak acid reacts with a strong base or vice versa, you have to know the molar concentrations of the two reactants. You’ll need to list the known dissociation constants of the reactants and set an ICE table for the reactants. ICE stands for ‘initial, change and equilibrium’, as shown in the example below.
 You’ll also need to know the concentration of the excess reactant. As a strong acid or base will completely dissociate and react, the excess reactant would be the weak reactant. You can then input the concentration of the excess into the pH equation.
You’ll also need to know the concentration of the excess reactant. As a strong acid or base will completely dissociate and react, the excess reactant would be the weak reactant. You can then input the concentration of the excess into the pH equation.











