Also known as haloalkanes and alkyl halides, halogenoalkanes are organic compounds derived from alkanes, but with one hydrogen substituted for a halogen. They’re part of a much larger category known as halocarbons. Hydrocarbons with more than one hydrogen substituted by a halogen are called halogenated hydrocarbons.
Halogenoalkanes have several commercial and industrial applications. For example, these compounds are commonly used in chemical manufacturing as the active components in flame retardants. Some halogenoalkanes are also used as refrigerants and propellants, while others are used in the synthesis of pharmaceutical products.
Some types of halogenoalkanes have serious environmental impact as pollutants. These have been gradually phased out, such as chlorofluorocarbons, or CFCs.
In this post:
What Are the Categories of Halogenoalkanes?
Halogenoalkanes are divided into three categories: primary, secondary, and tertiary.
These categories are derived from the number of alkyl groups attached to the carbon that carries the halogen substituent.
Carbon can form up to three covalent bonds with three other alkyl groups, and one covalent bond with a halogen atom.
- Primary halogenoalkane: Where only one alkyl group is attached to the carbon that has the halogen substituent.
- Secondary halogenoalkane: In this type, two alkyl groups are attached to the carbon with halogen. The alkyls may either be the same type or different types.
- Tertiary halogenoalkane: The halogen-carrying carbon has three alkyl groups directly attached to it. The alkyl groups are either of the same types or a combination of different types.
What Are the Properties of Halogenoalkanes?
Halogenoalkanes have very similar physical properties to the alkanes from which they’re derived. Just like their parent alkanes, haloalkanes are colourless, flammable, and some are hydrophobic.
Melting and Boiling Points
The melting and boiling points of some haloalkanes are significantly higher than their parent alkanes. This is especially true for those that have chlorine, bromine, and iodine atoms in them, provided that the halogenoalkane has the same carbon atoms as the parent alkane.
The main reason for their higher boiling points is the heavier atomic weights added to the alkanes by the halogen atoms. The intermolecular forces also significantly increase because of higher polarisability of the halogen component.
On the other hand, the boiling point of haloalkanes decreases in proportion to the number of branching increases. Isomeric haloalkanes have lower boiling points than their parent alkanes. One example of this is 2-bromopropane, which has a boiling point of 72.85°C. Its alkane analogue, 1-bromobutane, has a boiling point of 101.85°C.
Flammability and Polarity
Similar to their alkane parents, halogenoalkanes are flammable – but not as flammable as alkanes. Haloalkanes have fewer carbon-hydrogen bonds compared to alkanes, and the halogen atom makes halogenoalkanes slightly polar. Therefore, they’re significantly better as solvents compared to alkanes. Some halogenoalkanes are partially soluble in water.
Intermolecular Forces
The strength of the intermolecular forces, particularly the Van der Waals dispersion forces, is directly proportional to the length of the alkane molecules. As the halogenoalkane chain gets longer, the intermolecular forces become stronger. Longer chains require greater amounts of energy to break.
Similarly, the van der Waals dipole-dipole attractions also increase as the molecules become more polar because of the addition of halogen. The only exception is iodine because it has the same electronegativity value as carbon.
Chemical Reactivity
The chemical reactivity of halogenoalkanes is inversely related to the bond between the alkyl group and the halogen substituent. Halogenoalkanes with weaker bonds are more reactive than halogenoalkanes with stronger bonds. The bonding strength is directly proportional to the electronegativity of the halogen. This is the comparative electronegativity of halogens values of the halogens:
- F (4.0)
- Cl (3.0)
- Br (2.8)
- I (2.5)
What Are the Types of Reactions Halogenoalkanes Undergo?
The reactivity of halogenoalkanes with other compounds and elements is mainly determined by the halogen substituent. Haloalkanes have two main types of reactions: substitution and elimination. These main types also have five subcategories of reactions:
- Substitution reactions: These reactions involve the replacement of the halogen by another element. The general formula for substitution reactions can be written as:
R-X + Nu– → R-Nu + X–
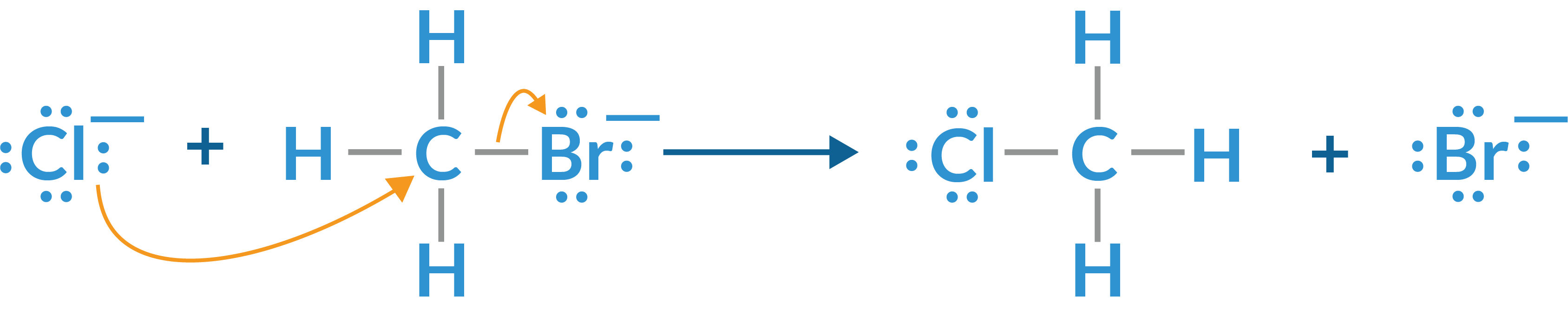
- Nucleophilic substitution: This reaction occurs when a nucleophile chemical donates a pair of electrons to an electrophile. The nucleophile replaces the electrophile in a compound. A more reactive halogen (a nucleophile) replaces a less reactive halogen. For example, the bromine in methyl bromide can easily be replaced by chlorine to form methyl chloride, as shown in the illustration below:

- Electrophilic substitution: This can be considered as the opposite of a nucleophilic substitution. An alkane substituent dislodges a hydrogen from an inorganic compound. An alkane substituent is deficient in electrons and may react with nucleophilic species, such as hydrogen halides, nitronium ions, and sulphur trioxide.
- Radical substitution: Free radicals are involved in this type of reaction. Halogen radicals may react with alkanes.
- Elimination/Dehydrogenation reactions: In this type of reaction, the halogen and an adjacent hydrogen (proton) are removed. An alkene is formed by this reaction.
What Are the Applications of Halogenoalkanes?
Halogenoalkanes have several commercial and industrial applications. Formerly, many of these compounds were used as refrigerants and fire retardants, but were later found to be serious environmental threats. Here are some other applications of haloalkanes:
- Pharmaceutical products: Many pharmaceutical products, including some top drug brands, have alkyl fluoride compounds. Some well-known brands include the anaesthetic Rohypnol and the antidepressant Prozac.

- Industrial solvents: These are used to dissolve many types of substances and process them into different products like teflon. Examples include chloroform, dichloromethane, dichloroethene, and trichloroethane.
- Fumigants: Alkyl bromides are largely used as fumigants to kill pests, such as some species of insects and nematodes.
For more A level chemistry resources to help you revise and for information on studying chemistry A level, check out our A Level Chemistry Resources hub.