The A level organic chemistry syllabus includes the study of amino acids, proteins and DNA. To help you prepare for your exam, we’ve put together a summary of the key points you need to revise.
In this post:
A brief timeline
The relationship between amino acids, proteins and DNA is a relatively recent discovery. It wasn’t until 1944 that the link between heredity and DNA was first outlined. Before that, scientists thought proteins served as the vehicle that carried the information for genetic inheritance.
The chromosomes found inside the nucleus of cells are actually tightly-packed DNA molecules. These molecules contain the instructions for synthesising proteins from amino acids. The codes in DNA molecules are composed of codons (groups of three nucleotides) that correspond to specific amino acids. Amino acids are assembled based on the sequence of codons that are switched on during protein synthesis.
Here’s a timeline of discoveries related to amino acids, proteins, and DNA.
- 1806 – the first amino acid, asparagine from asparagus, was isolated by French chemists Louis-Nicolas Vauquelin and Pierre Jean Robiquet.
- 1838 – Swedish chemist, Gerardus Johannes Mulder, chemically described proteins and named them based on the suggestion of Jöns Jacob Berzelius. Mulder also identified the products of protein degradation, such as the amino acid leucine, thereby establishing the connection between amino acids and proteins.
- 1869 – the organic chemical then known as “nuclein” was isolated from a cell nucleus by Friedrich Miescher. This was later named DNA.
- 1881 – German biochemist, Albrecht Kossel, identified nuclein as nucleic acid. He was also able to isolate the five nitrogen bases that compose DNA and RNA.
- 1882 – Walther Flemming systematically studied chromosomes during cell division. His studies would later become significant in the study of genetic inheritance.
- 1900s – Theodor Boveri and Walter Sutton independently developed a theory of chromosomes as the basis for genetic inheritance.
- 1940s – Barbara McClintock made a breakthrough discovery about the mobility of genes in chromosomes. This discovery earned her the Nobel Prize in Physiology in 1983.
- 1944 – Oswald Avery outlined the facts supporting the idea that DNA is the basis for cell properties and not proteins as previously thought.
- 1944-1950 – Erwin Chargaff conclusively demonstrated that DNA is the molecule responsible for passing down genetic information from parents to offspring. He also proved that organisms have varying DNA patterns, especially among different species.
- 1951 – English chemist Rosalind Franklin worked out the molecular structure of DNA based on X-ray crystallography images. This became the foundation of the detailed work of Watson and Crick. Unfortunately, Rosalind’s contribution to the discovery of DNA structure was only acknowledged after she died.
- 1953 – Watson and Crick published their findings on the double helix structure of DNA. It was based on the X-ray crystallography work of Rosalind Franklin. This earned them the Nobel Prize in Physiology or Medicine in 1962.
What are amino acids?
Amino acids are called the “building blocks of proteins”. They form polymer chains, which determine the type of proteins. The Practical Handbook of Biochemistry and Molecular Biology contains more than 300 amino acids, but only 20 have roles in the synthesis of proteins in organisms.
Of the 20 amino acids in the human body, nine are considered essential as they can only be derived from food. The remaining 11 amino acids are deemed non-essential because they can be synthesised by the body.
The various amino acids can combine in a myriad of ways, forming proteins that either have structural roles, such as the skeletal muscles, or physiological-biochemical roles such as enzymes. According to some estimates, the body can synthesise several billion types of proteins from amino acids.
Types of amino acids
The general structure of amino acids consists of a basic amino group, an acidic carboxyl group, and an organic chain or ring designated as the R group.
Amino acids can be grouped into four categories based on the chemical nature of the R group:
- Non-polar amino acids
- Polar amino acids
- Positively-charged amino acids
- Negatively-charged amino acids.
The names of amino acids can be abbreviated into three letters, as shown in the tables below. In this manner, it would be easier to create a table with codons corresponding to specific amino acids.
What are zwitterions?
Some amino acids can exist as zwitterions when dissolved in water. A zwitterion is an ion with two functional groups. The ion’s charge is directly related to the pH level of the solution. Amino acids that have uncharged R groups exist as cations in acidic solutions. The same amino acids become anions in basic solutions. 
Optical activity of amino acids
The optical activity of an amino acid refers to its alpha carbon, which is the chiral centre of the molecule. Amino acids are asymmetric molecules, meaning there are variations in their mirror image. This is known as chiral isomerism. As an amino acid can be left-handed or right-handed, it’s not superimposable with its mirror image. 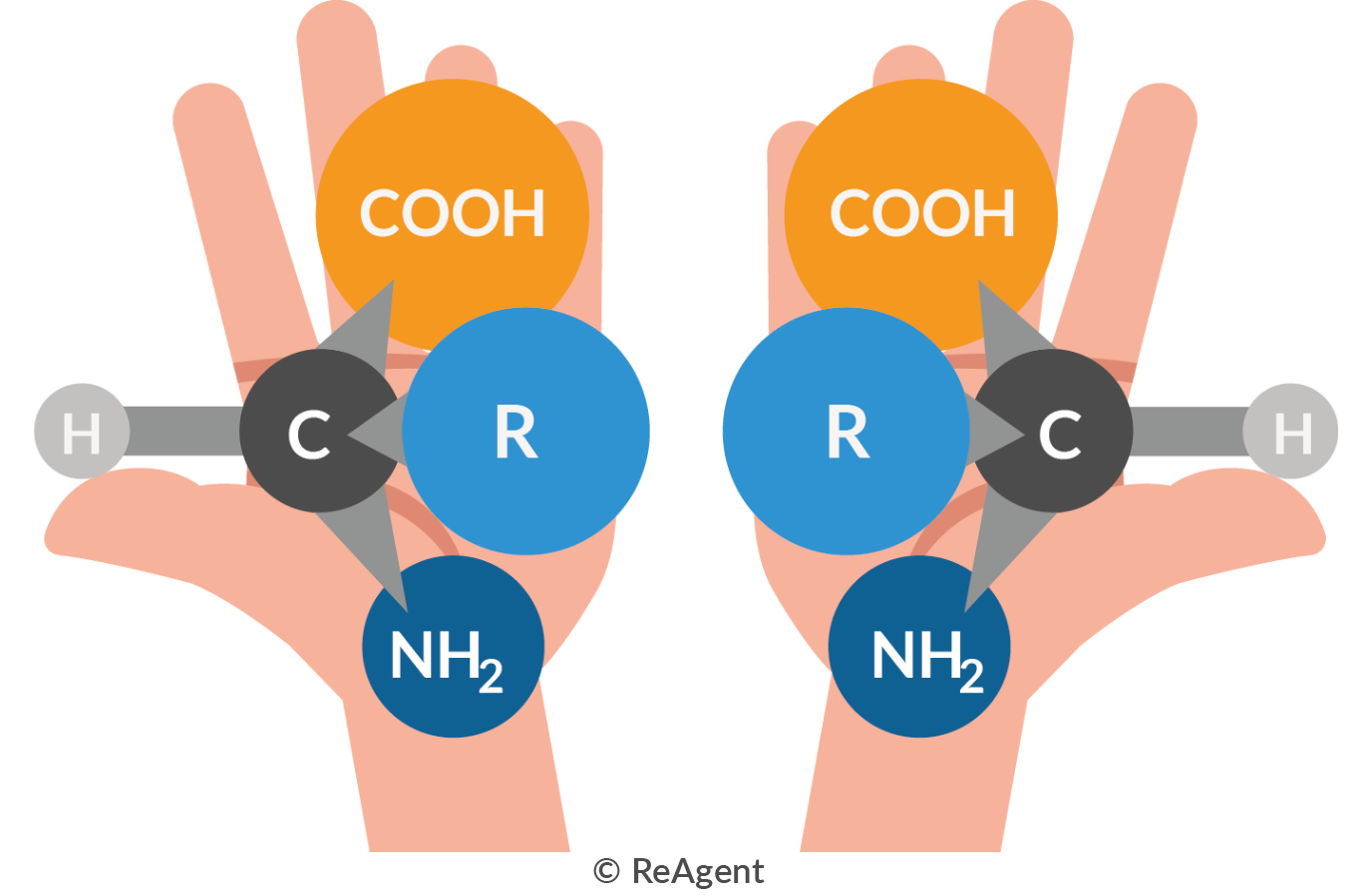
As you can see in the above illustration, if you try to flip the mirror image the two molecules cannot be superimposed on each other. This is comparable to the spatial relationship between your left hand and your right hand.
What are proteins?
Proteins are complex biological molecules composed of chains of amino acids. They are the products of genetic coding instructions. The constituent amino acids are bonded together by peptide bonds, which are a type of covalent bond. The peptide bonds in proteins are the result of the attraction between the carboxyl group of one amino acid and the amino group of another amino acid.
Proteins can be divided into the following seven categories, depending on their roles in an organism:
- Antibodies
- Contractile proteins
- Enzymes
- Hormonal proteins
- Structural proteins
- Storage proteins
- Transport protein
Peptide bonds and polypeptides
Every amino acid in a protein is bonded to another amino acid through peptide bonds. They can form various types of proteins depending on the sequence of amino acids, the number of amino acids and their structure. A long chain of amino acids is called a polypeptide chain. 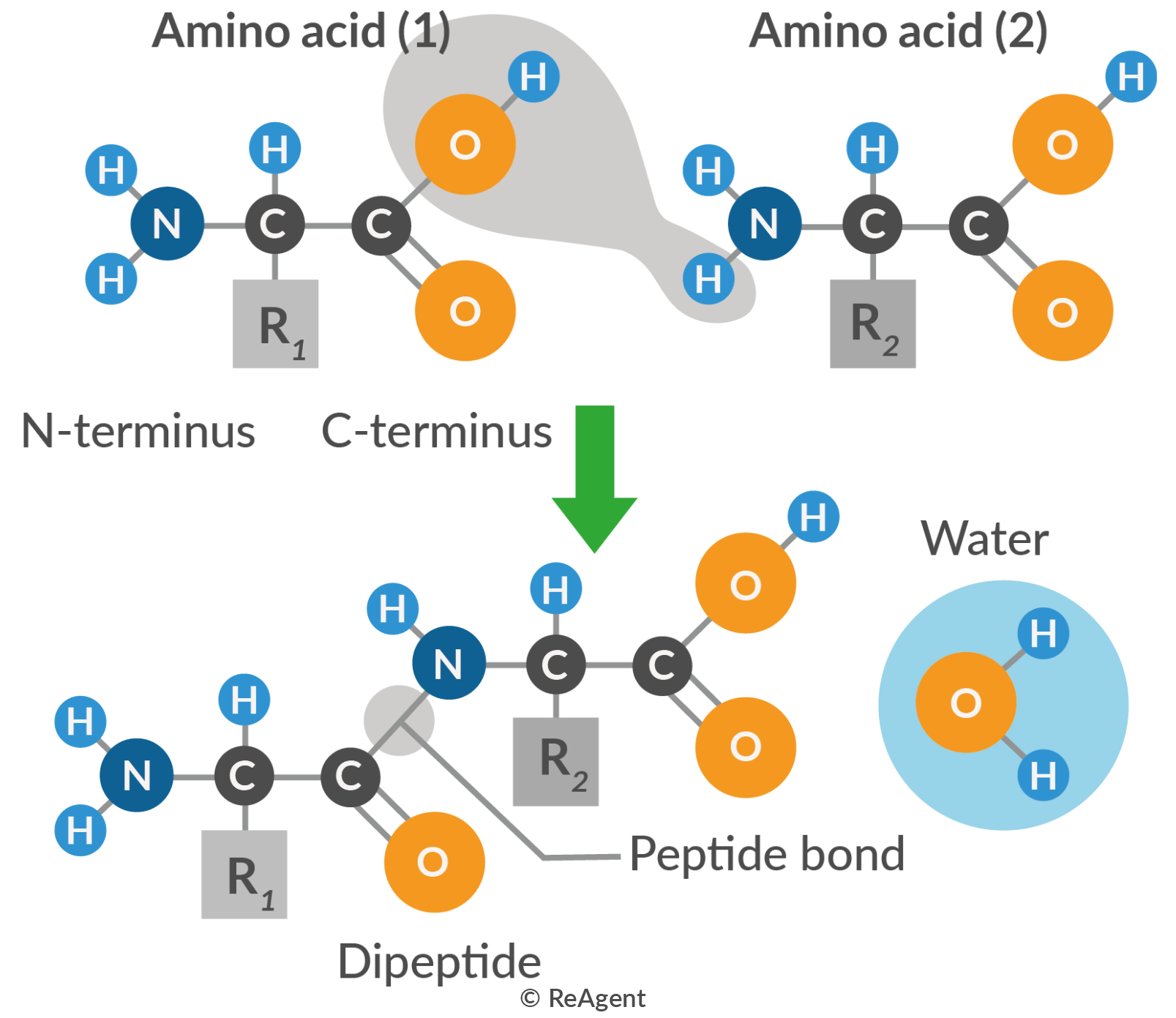
Structure of proteins
The roles and capabilities of proteins vary depending on their molecular structure. For example, antibodies or immunoglobulins (IgG, IgA, IgD, IgE, and IgM) change their shape or structure in response to infection. The body develops immunity by remembering the corresponding protein structures of pathogenic microbe invaders.
Proteins can form four types of structures:
- Primary structure – this refers to the polypeptide chains of amino acids
- Secondary structure – when the polypeptide chain bends or folds, it forms a secondary structure, which can either be α-helix or β-sheets
- Tertiary structure – when the R groups in a polypeptide chain interact with each other through intermolecular forces (for example, hydrogen bonds and van der Waals forces), they form a tertiary structure
- Quaternary structure – when more polypeptide chains interact with each other through hydrogen bonds, covalent bonds and hydrophobic interactions, they form a quaternary structure
What is DNA?
DNA, or deoxyribonucleic acid, is a polymer composed of nucleic acids. It’s often called the blueprint of life because it contains the instructions on how to create an organism and maintain its functions. Almost all organisms have DNA as their genetic material. Others, especially some microorganisms, use RNA (ribonucleic acid) as their genetic material.
In complex multicellular organisms like humans, DNA molecules are contained within the nucleus of the cells. They are packed into chromosomes during cell division. Sexual and asexual cell division allows for some mixing of genes that results in variations in individual organisms. Coupled with random mutations, DNA replication becomes a main driving force in the evolution of a species.
Structure of DNA
Through x-ray crystallography analysis, scientists discovered that DNA has a double helix structure, similar to that of a ladder. It has four types of nucleotides that are paired and bonded together through hydrogen bonds. The pairing is as follows:
- Adenine is paired with thymine
- Guanine is paired with cytosine.
In RNA transcription, uracil is the analogue of thymine, which pairs with adenine. 
Cellular location and replication
The cellular location of DNA is the nucleus. It replicates during cell division (meiosis or mitosis) as the cell splits into daughter cells. The DNA double helix unzips, the template is primed and then a new DNA molecule is assembled. The long strands of DNA are compacted into chromosomes.
DNA sequence
The way the nucleotides are arranged into sets or sequences corresponds to the genetic codes. Several genes may simultaneously be switched on or off, depending on what a cell needs. The instructions to create proteins depend on the DNA sequence.
For more guides to help you prepare for your A level chemistry exam, visit our A level resources hub.













