Ethanoic acid is another name for acetic acid, but it’s more popularly known as the active ingredient in vinegar. The most typical example of a carboxylic acid, ethanoic acid has an acidic smell and taste, and is used as a preservative because its acidic environment is inhospitable for bacteria. But what is the real chemistry behind this chemical?
In this post:
Key Takeaways
Ethanoic acid is more commonly known as acetic acid, which is the key ingredient of vinegar
Unlike vinegar, the industrial-scale mass production of ethanoic acid does not involve a fermentation process
Ethanoic acid is an organic chemical with the formula C2H4O2
Ethanoic acid has many uses, such as acting as a chemical precursor in manufacturing synthetic polymers
Household vinegar is approximately one molar (1.0M) solution of ethanoic acid. It has an acidity of about pH 2.4
Introduction to Ethanoic Acid: Overview & Properties
Industrially-produced ethanoic acid is the product of a forced chemical reaction between methanol and carbon monoxide. The acid is produced under high pressure and temperature of 30 atm (3,039.75 kPa) and 450K (176.85 °C), respectively. A rhodium/iodine based catalyst system is used in the process.
Ethanoic acid is a weak organic acid, which means it does not completely dissociate into ions when dissolved in water. It has several industrial applications, such as the production of synthetic polymers. It’s also used in the food industry as a food flavouring and preservative. Ethanoic acid is the active ingredient in vinegar.

Common Name and Formula of Ethanoic Acid
The most common name is actually ethanoic acid (CH3COOH), and this is also the systematic name assigned by the International Union of Pure and Applied Chemistry (IUPAC). This chemical is also known by formal and informal names, including:
- Acetic acid
- Methanecarboxylic acid
- Hydrogen acetate
- Ethylic acid
The systematic name, ethanoic acid, is the more precise name because it contains information about this chemical’s functional group and structure. As we’ve said, ethanoic acid is the active ingredient in vinegar, but don’t mistake the two for the same thing. Vinegar is a dilute aqueous solution of CH3COOH, and many impurities are present in it to make it fit for human consumption. So, vinegar technically isn’t ethanoic acid – it’s just a 4-8% solution of it.
What Is The Formula Of Ethanoic Acid?
The chemical formula of ethanoic acid, or acetic acid, can be written as C2H4O2. This chemical formula indicates the respective proportional numbers of elements per molecule. In this case, it simply means that for every molecule of ethanoic acid, there are two atoms of carbon, four atoms of hydrogen, and two atoms of oxygen.
However, there are other ways to write the formula of ethanoic acid in order to provide more insights into its chemical properties and other attributes. For instance, it’s commonly written as CH3COOH, a functional group formula indicating that there are two groups in one molecule: the CH3 is the methyl group while the COOH is the single carboxyl group.
For chemists, the various formulae for ethanoic acid have corresponding value in terms of interpreting the chemical behavior of this substance. The methyl group, for example, is common among hydrocarbon chains. This implies that ethanoic acid can be derived using hydrocarbons, or alcohols like ethanol. In fact, ethanoic acid is produced when ethanol is oxidised. The carboxyl group, on the other hand, indicates that this substance can be deprotonated (have its protons removed), which is what makes it acidic and corrosive.
The functional group formula of ethanoic acid can also help predict the possible reactions it has with other substances. For example, when ethanoic acid is dissolved in water, positive and negative ions are formed, but the methyl group and the carboxyl group don’t dissociate completely. Instead, the following happens:
CH3COOH + H2O ⇆ H3O+ + CH3COO– As you can see, the carboxyl group donates an extra proton (hydrogen nucleus) to the water molecule. This makes the ethanoic ion negatively charged. As an acid, albeit a weak one, it donates a proton or a positively charged hydrogen ion when it reacts to a base. The weak acidic properties of this chemical means that it has a number of wide-ranging applications, including:
- As a precursor for cellulose acetate, which is used in photography and printing
- As a precursor for polyvinyl acetate for manufacturing synthetic fibres
- As a component of sealants like wood glue
- As a degreasing solvent – you may have heard of the miracles of cleaning with vinegar
- As an antibiotic for bacterial or fungal infections
- As a food preservative because of its ability to stop bacterial growth
What Is The pH Value Of Ethanoic Acid?
The pH of ethanoic acid varies depending on its concentration. For instance, at a concentration of 1.0 M solution, which is the approximate concentration of household vinegar, the pH is 2.4. This is an indication that only 0.4% of the molecules of the acid are dissociated.
Alternatively, at a very low concentration of only 10-6 M solution, about 90% of the molecules are dissociated. The pH level of any dissolved substance demonstrates its tendency to either donate protons, making it acidic, or accept protons, making it alkaline. The protons here refer to the nucleus of the hydrogen atom in a solution at a particular concentration.
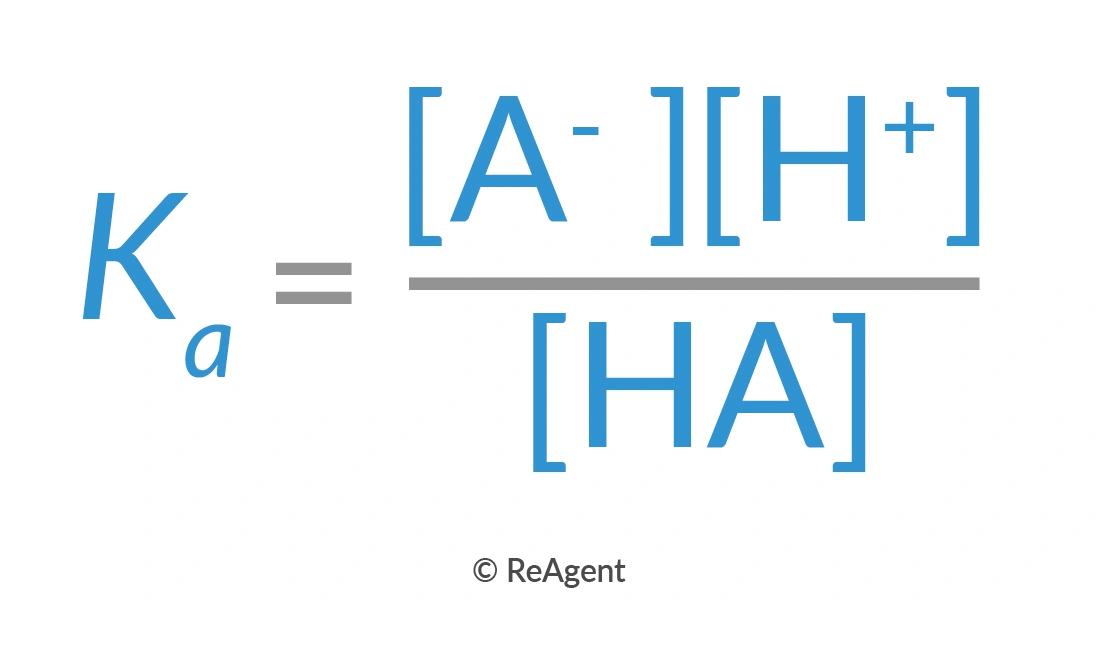
That’s why pH stands for ‘potential of hydrogen’. pH is calculated as the concentration of hydrogen ions, but in negative logarithmic form, with a range of 0 to 14, making pH 7 a neutral reading. While acids have pH levels less than seven, bases or alkaline solutions have pH levels higher than seven. But pH isn’t the only way to measure the weakness or strength of an acid or base. The true measure of acidity or alkalinity strength is the dissociation constant. This is calculated based on the formulas below:
 Alternatively expressed as:
Alternatively expressed as: 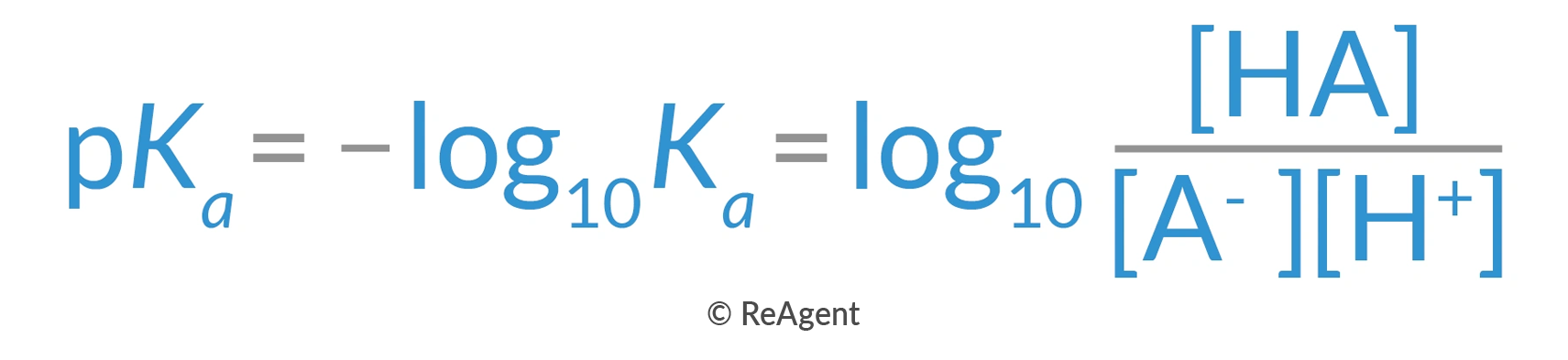 Basically, the dissociation constant is the proportion between the number of intact molecules of a substance in a solution and the ions that are dissociated. Ethanoic acid has a dissociation constant of Ka=1.7×10−5 or pKa=4.76. The logarithmic version is used to simplify the number by removing the scientific notation. The main reference is the hydronium ion, or H3O+, which has a Ka of 1.00 and a pKa of 0.00.
Basically, the dissociation constant is the proportion between the number of intact molecules of a substance in a solution and the ions that are dissociated. Ethanoic acid has a dissociation constant of Ka=1.7×10−5 or pKa=4.76. The logarithmic version is used to simplify the number by removing the scientific notation. The main reference is the hydronium ion, or H3O+, which has a Ka of 1.00 and a pKa of 0.00.
What Colour Does Ethanoic Acid Turn On Blue Litmus Paper?
Ethanoic acid turns blue litmus paper red. This makes sense when you consider that, in a litmus paper test, blue paper is used to test for acidity while red paper is used to test for basicity.
However, litmus paper is only used as an indicator test; it cannot provide precise readings of the pH level of a solution. Litmus paper has a pH range of 4.5–8.3 at a temperature of 25°C. This means that blue litmus paper will turn red if the pH is at 4.5 or below.
Conversely, red litmus paper will turn blue if the pH level is at 8.3 or above. If the litmus paper turns purple in either case, this means that the pH level is near neutral. A litmus test is a common laboratory technique for determining whether a substance is acidic or alkaline.
Since ethanoic acid has a pH level below 4.5, it’s still easy to detect using litmus paper. However, because of the limited range of litmus paper, it may not be able to detect the acidity of ethanoic acid at certain levels. A more accurate instrument to use for precise readings is a digital pH meter. Litmus tests are rarely performed in analytical chemistry, especially in industrial laboratories.
This is because it doesn’t provide a precise enough reading. However, it’s still commonly performed in secondary school laboratory studies as proof of concept.
Is Ethanoic Acid a Strong or Weak Acid?
A 1.0M concentration of ethanoic acid dissolved in water has a pH level of 2.4, so only about 0.4% of the molecules of the acid dissociated into ions. This means that ethanoic acid is a weak acid by definition.
Uses of Ethanoic Acid in Industry & Daily Life
Ethanoic acid has several practical applications in industries and in daily life. It’s indispensable in several manufacturing processes, as well as being used in households.
Industrial Applications of Ethanoic Acid
Ethanoic acid is a precursor chemical for various industrial products. For example, it’s a precursor for cellulose acetate, which is used in photography and printing.

Household and Food Industry Uses
Ethanoic acid is the active ingredient of vinegar, which is an important ingredient in many dishes. It’s used a flavouring that also helps preserve food because of its antimicrobial properties. For thousands of years, people have been using vinegar for food preparation and also for treating certain common ailments.
Safety & Handling of Ethanoic Acid
Ethanoic acid has relatively low toxicity even at high concentrations. Nonetheless, it can cause skin irritation, and inhaling its vapours can result in lung problems. There are also some potential environmental impacts of ethanoic acid when it’s improperly disposed of.
Precautions When Handling Ethanoic Acid
When you handle highly concentrated ethanoic acid, you should wear standard PPE, such as gloves, goggles, and a facemask or breathing apparatus. It’s also important to avoid skin exposure or inhalation of the vapour.
Environmental Impact & Disposal
If neutralisation of large amounts of ethanoic acid isn’t possible, you must ensure you don’t directly pour it onto the ground or contaminate natural sources of water. The pH balance of soil might be adversely affected and some aquatic organisms may suffer. It’s very important to treat any acidic waste chemically before you dispose of it.
Conclusion
Ethanoic acid is a weak acid that’s naturally produced through fermentation. However, industrial ethanoic acid is produced through a forced reaction between methanol and carbon dioxide, under high pressure and temperature, and using a catalytic system. It has several household and industrial applications. It might not be highly toxic, but proper precautions should be taken to prevent injury and environmental impact.












