If you want to go on to study chemistry or science at university, you’ll first need to pass chemistry GCSE. Passing the GCSE chemistry exam isn’t only about memorising chemical names, it’s about understanding and applying deeper concepts in chemistry.
Chemistry GCSE will make you more proficient in analytical logical thinking, and will provide you with a good foundation of knowledge for pursuing a chemistry degree or a career in chemistry. Some schools even offer revision programs for GCSE chemistry intended for adults who want to pursue higher education and different or improved career opportunities.

Why Study Chemistry GCSE?
Passing GCSE chemistry can open up opportunities. While it may not be the only qualification you’ll need to be successful in the scientific field, it will provide you with a solid foundation to get started. For example, studying chemistry GCSE will give you the foundational knowledge needed to succeed at A level chemistry.
Similarly, you’ll have a better chance of getting accepted by the university of your choice if you study and excel at chemistry GCSE. Then, once you’ve passed A levels (or equivalent) and have earned your chemistry degree, you’ll be able to begin pursuing your dream chemistry career. All of this is made possible by studying chemistry GCSE, the first domino that will set the rest of your future in motion.
Various career opportunities are available to you when you have a successful chemistry education. Here are some of the best careers in science and chemistry you can pursue:
- Chemical engineer
- Chemistry teacher or professor
- Cosmetic chemist
- Forensic scientist
- Geochemist
- Materials scientist
- Pharmacologist
- Pharmaceutical chemist
- Toxicologist
If you have a strong knowledge of chemistry combined with business acumen, you may also be able to form your own business and develop your own products. From cosmetics and food products to medicines and biotechnology, chemistry is essential in developing and improving a wide range of products for the betterment of humanity.
Whatever path in chemistry you choose, you will have an exciting, challenging, and rewarding career. You’ll be exposed to state-of-the-art technologies and trends in your field, and will have the opportunity to work with some of the most brilliant minds in the world.
What Are the Topics Covered in GCSE Chemistry?
- Atomic Structure and the Periodic Table
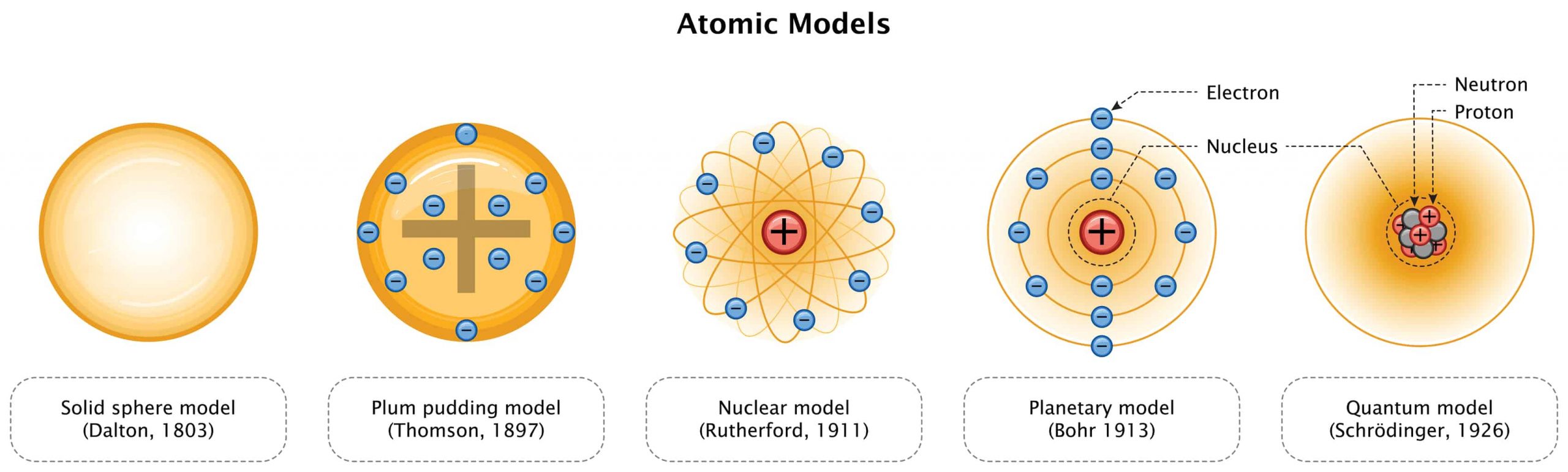
The ancient Greek philosopher, Democritus, is credited with the development of the idea about the atom. He proposed a fundamental indivisible particle that composes everything. This became one of the bases for trying to understand the nature of matter. The modern atomic theory is based on various mathematical and experimental data that indicate a model of the atom.
The current atomic model is a synthesis and improvement of various models. Its accuracy is supported by empirical evidence and other related scientific discoveries, particularly in quantum mechanics. Basically, the atomic theory can be paraphrased as:
- At the center of an atom is a nucleus
- A nucleus is typically comprised of protons and neutrons
- The nucleus is surrounded by a cloud of electrons
The chemical elements have a continuous pattern that can be arranged in a periodic table based mainly on the atomic number, i.e. the number of protons. The Russian scientist, Dmitri Mendeleeev, was the first to develop the law of periodicity of the elements. He saw gaps in the table and predicted other elements that were yet to be discovered.
In chemistry GCSE, you will develop your knowledge on this topic, learning the history of the periodic table, the structure of the atom, the properties and atoms of the chemical elements, and how they interact with each other.
- Bonding, Structure, and the Properties of Matter
Chemical reactions are fundamentally about bonds. They either involve the breaking of bonds, the creation of bonds, or both. The electrons in the outer shells of atoms are the ones involved in chemical bonds. Meanwhile, the properties of the compounds formed by chemical reactions are determined by the elements, specifically by the number of protons.
The molecular structures, including the isomeric positions, also contribute in determining the properties of matter. For example, the chemical formula C2H6O represents two different isomers, namely, methyl ether and ethanol.
In your GCSE lessons, you’ll learn about all the different types of bonds, including chemical bonds, ionic bonding, ionic compounds, covalent bonding, and metallic bonding. The structure and bonding of carbon will also be taught, specifically in regards to diamond, graphite, graphene, and fullerenes.
For properties of matter, topics you’ll study include:
- States of matter (solids, liquids, gases)
- State symbols, e.g. (s) meaning solid, (aq) meaning aqueous solutions
- Properties of ionic compounds
- Properties of small molecules
- Giant covalent structures, including polymers
- Properties of metals and alloys
The size and uses of nanoparticles will also form part of your learning at chemistry GCSE. The knowledge you gain from this specific topic will be crucial if you’re interested in a career in nanotechnology or nanoscience, a field that is at the epicentre of human improvement thanks to its focus on revolutionising technology and industry sectors.
- Quantitative Chemistry
Studying chemistry requires precise and accurate measurements. Using various instruments to measure specific parameters is crucial in predicting, analysing, and synthesising chemical compounds.
From using simple instruments, like a graduated cylinder, to using expensive and sophisticated instruments, like gas chromatography equipment, you need to develop the skills of calibrating your instruments and making precise measurements.
At GCSE, the topics you’ll cover on quantitative chemistry will strengthen your competency in making precise measurements by covering key topics like:
- The Law of Conservation of Mass
- How to balance chemical equations
- How to calculate the relative formula mass of a substance
- Chemical measurements and amounts
- Limiting reactants
- Measuring the concentration of solutions
- Atom economy and yield
- How to calculate gas volumes

- Chemical Changes
Determining or distinguishing between a physical change and a chemical change is sometimes difficult to do. Some common indicators of a chemical change that you’ll study at GCSE are:
- Change in colour
- Evolution of a gas, or formation of bubbles
- Change in temperature (exothermic or endothermic)
- Emission of light
- Formation of precipitates
- Energy Changes
In chemistry GCSE, you’ll also study energy changes in chemical reactions, such as in exothermic and endothermic reactions.
For example, you could undertake lab experiments to study how various types of metals, like magnesium, potassium, sodium, zinc, iron, lithium, calcium, and copper, react with water, or with a dilute solution of an acid. One key thing you’ll measure here is the differences in temperature change, indicating whether an exothermic or endothermic reaction has occurred.
You’ll also learn how to create and read reaction profiles in order to understand and show the energy changes that take place during reactions.
- The Rate and Extent of Chemical Change
An important area of study will be the various factors that affect the rate of chemical reactions. These are either environmental factors or factors that are innate to the properties of substances. For example, sodium is more reactive than iron because of the structure of the valence electrons.
Other factors you’ll study include:
- Temperature
- Pressure
- Presence of a catalyst
- Saturation or concentration

- Organic Chemistry
Organic chemistry deals with various types of compounds that have carbon as the central element. It has some overlaps with biochemistry, but the main focus of the study are compounds that can be synthesised outside the body or cell of a living organism.
Studying organic chemistry at GCSE is focussed on five classes of organic compounds and their general structures and functional groups:
- Hydrocarbons: These are compounds that contain only carbon and hydrogen. They include the branched hydrocarbons and the aromatic or cyclic hydrocarbons
- Alcohols and ethers: These compounds can be thought of as derivatives of water in which one or two of the hydrogen atoms have been replaced by an organic chain. The structural formulas of these two types of compounds are illustrated below:
- Aldehydes and ketones: Many examples of these compounds have strong and distinct aromas. Aldehydes are the major components of food flavourings, like vanilla and cinnamon. Similarly, many ketones are components of compounds with strong aromas, like camphors and jasmine. They’re also found in many sex hormones. Both aldehydes and ketones have carboxyl functional groups:
Ketones general formula:
Aldehydes general formula:
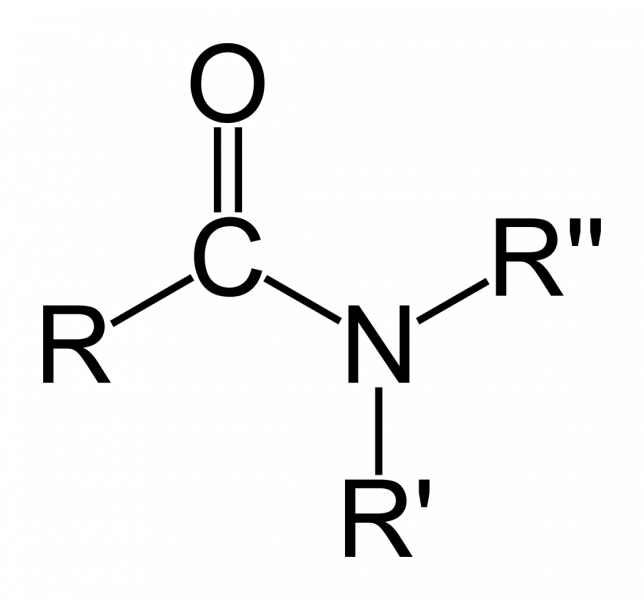
- Amides: These compounds have substituents that are attached to nitrogen. They have peptide bonds that can form long chains, and are constituents of proteins and synthetic materials, like nylons:
Amides general formula:
- Amines: These are organic compounds that are derived from ammonia. They contain nitrogen atoms with a lone pair. A hydrogen atom is replaced by an alkyl or aryl group. Amines are used in synthesising drugs and crop protection chemicals:
General formula of amines:
- Chemical Analysis
Chemical analysis is central to the study of chemistry, and is a key topic in the GCSE syllabus. Virtually all concepts and experiments involve analysis. Chemical analysis is empirical and typically done in the laboratory using various instruments and methodologies, such as titration or chromatography.
The main objective is to isolate each functional component in order to understand the whole. Chemical analysis is the process of breaking down a substance into smaller parts or groups so that you can understand its properties and composition.
This topic has environmental and ecological significance. In a nutshell, you’ll study atmospheric composition and dynamics in order to understand how greenhouse gases have an impact on climate change and global warming.
For instance, you’ll study the different gases that create the atmosphere, how these components have changed over time, and how CFCs (chlorofluorocarbons) react with ozone.
Studying all of these topics at chemistry GCSE will better prepare you to handle the concepts introduced at A level. Also, by creating a sturdy foundation of knowledge at GCSE, you’ll be able to set yourself up for success at university and beyond.
For more help and support on revising for GCSE chemistry, read our revision series:
- Chemistry GCSE Revision: Atomic Structure And The Periodic Table
- Chemistry GCSE Revision: Properties of Matter
- Chemistry GCSE Revision: Quantitative Chemistry
- Chemistry GCSE Revision: Energy Changes
- Chemistry GCSE Revision: The Rate and Extent of Chemical Change
- Chemistry GCSE Revision: Organic Chemistry
- Chemistry GCSE Revision: Chemical Analysis
- Chemistry GCSE Revision: Chemistry of the Atmosphere
- Chemistry GCSE Revision: Using Resources
- Chemistry GCSE Revision: Practical Skills