Among the fundamental concepts in GCSE chemistry that you should revise is the atomic structure and the arrangement of elements in the periodic table.
This article will provide some guidelines and tips on how to effectively understand this important topic in preparation for your GCSE exam. Links to external sources are provided for more in-depth revision.
In this post:
Atoms, Elements And Compounds
All normal matter that we encounter, measure, observe, and manipulate – including the atoms in our own bodies – is baryonic matter.
A baryon is a subatomic particle that is composed of an odd number of valence quarks. Baryons aren’t only classified under the hadron family of particles, they’re also classified as fermions based on their half-integer spin. While this is a well-known type of matter, it’s also actually the least abundant organised matter.

Baryons are what dark matter is composed of. All the stars, planets, interstellar gases, and black holes in the universe are less than 5% of the total measurable mass of the universe.
In comparison, dark matter makes up about 27% of the observable universe. Although these facts aren’t in the province of chemistry, or directly relevant to a chemistry degree, it’s important to be aware that chemistry is about normal matter or baryonic matter.
What Are Atoms?
Atoms are the fundamental baryonic particles that define the properties of elements and compounds. The atomic number, which is the number of protons an atom contains, acts as the identity of an element. For instance, the simplest element, hydrogen, has an atomic number of one. Meanwhile, the heaviest naturally occurring element, uranium, has 92 protons and, in turn, the atomic number 92.
What Happens When Atoms Get Heavier?
As elements become heavier, they tend to become less stable, making them radioactive. Some elements that have lower atomic numbers also have radioactive isotopes, like carbon-14.
What Are Isotopes?
An isotope of an element is either heavier or lighter because of the presence or absence of extra neutrons. All elements in the periodic table between 1 (hydrogen) and 82 (lead) have at least one stable isotope, with the exception of two elements: technetium (43) and promethium (61).
Do Elements Naturally Occur in Pure Form?
Most elements don’t occur in pure form in nature: they’re combined with other elements to form compounds. However, noble gases, i.e. inert gases, don’t easily combine with other elements. The noble gases are:
- Helium (He)
- Neon (Ne)
- Argon (Ar)
- Krypton (Kr)
- Xenon (Xe)
- Radon (Rn)
Because they don’t combine with other elements well, these gases all naturally occur as pure elements.
Here is a comprehensive guideline about the topics to revise regarding atoms, elements and compounds. It includes chemical formulae of elements, ions, some common compounds, and balanced chemical equations. You may also want to try answering the questions about compounds here.

Mixtures
Mixtures are physical combinations of two or more different substances. They are either homogeneous (where the mixture is uniform throughout) or heterogeneous (non-uniform). The chemical properties of substances are retained.
The dispersion media of mixtures, also called the mixture phase, can be gas, liquid, or solid. For instance, table salt (solid) can be dissolved in water, which acts as the dispersion medium.
Mixtures are classified into three categories:
- Solution: This is a type of mixture in which one or more solutes are dissolved in a solvent to form a homogeneous substance. The solute is typically less in amount compared to the solvent. Every solution has a saturation point, which is the limit by which no further amount of solute can be dissolved in the solvent.
- Colloid: This is a phase-separated type of mixture in which insoluble or soluble particles are microscopically dispersed throughout another substance. Colloidal mixtures include gels and emulsions. Particles don’t settle in colloid mixtures and cannot be separated through ordinary filtration or centrifugation.
- Suspension: This is a heterogeneous mixture in which the particles are large enough to be separated by filtration or centrifugation. When left for a period of time, the particles settle down the bottom of the dispersion medium, like mud suspended in water.
Check out these revision questions about mixtures to help you prepare for your GCSE chemistry exam.
Atomic Structure
Atomic structure refers to how the subatomic particles comprising an atom are arranged. You may find it useful to revisit the history of how scientists John Dalton and J.J. Thomson theorised the structure of the atom.
Knowing the origin of the atom will help you understand how the idea evolved from the discoveries about protons, neutrons, and the arrangement of electrons. You should also revise the various experiments of Bohrs and Rutherford and how calculations were made to come up with the idea that electrons orbit the nucleus in shells.
The modern quantum mechanical model of the atom puts the electrons in probabilistic clouds where they exist as wave-particles, occupying regions called orbitals. The electrons are quantised in this model, allowing them to exist only on certain energy levels and not in between.

The Periodic Table
Even before Dmitri Mendeleev arranged the known chemical elements in an orderly table in 1869, there were already some inklings that the elements followed certain patterns and groupings. An example of the type of patterns and groups that elements follow are noble gases: these are all inert and do not easily form compounds with other elements in ordinary conditions.
The periodic table of elements is simply the logical arrangement of the elements based on their properties. It is not sufficient to simply arrange the chemical elements using their atomic numbers. The columns and rows classify elements into categories and transitions. For example, the first column or the hydrogen group are the alkali metals. They form alkaline substances when they react with water.
Refer to the sample questions on this page to test yourself on the periodic table of elements.
Groups In The Periodic Table
The periodic table of elements has 18 groups that correspond to each column.
Each group is represented by the element on top of the column, which shares similar physical and/or chemical properties with the other elements in the group. This is mainly because of the electron shells of their atoms. You must remember that the reactivity and bonding tendencies of the elements are largely determined by the orbital location of the outermost electron.
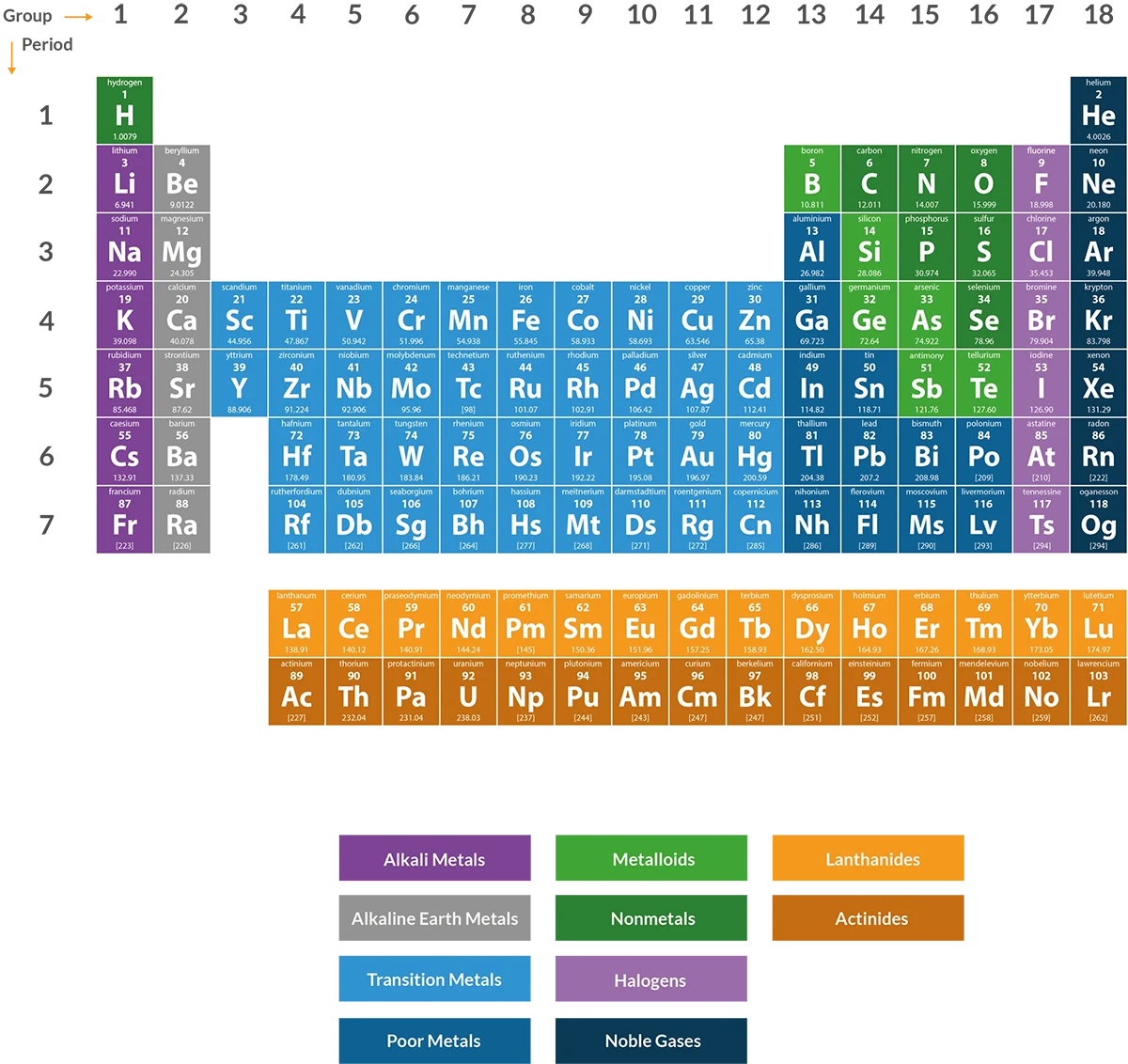
Transition Metals
The transition metals are the elements found right in the middle of the periodic table of elements. Specifically, they belong to Groups 3 through to 12. They are elements that have valence electrons in two separate shells instead of just one. This is significant in terms of how they combine with other elements that belong to other groups.
Refer to this resource for further information and sample GCSE questions about transition metals.
Find out more about chemistry education in our chemistry education resources hub.
For more help and support on revising for GCSE chemistry, read our revision series:
- Chemistry GCSE Revision: Properties of Matter
- Chemistry GCSE Revision: Quantitative Chemistry
- Chemistry GCSE Revision: Energy Changes
- Chemistry GCSE Revision: The Rate and Extent of Chemical Change
- Chemistry GCSE Revision: Organic Chemistry
- Chemistry GCSE Revision: Chemical Analysis
- Chemistry GCSE Revision: Chemistry of the Atmosphere
- Chemistry GCSE Revision: Using Resources
- Chemistry GCSE Revision: Practical Skills











