Isopropyl alcohol, which is also known as isopropanol and propan-2-ol, comes in different grades and has a large variety of applications in many different industries.
In this post:
Key Takeaways
Isopropyl alcohol has many uses; one of which is rubbing alcohol
Ethyl alcohol is also used as rubbing alcohol
Isopropyl alcohol is commonly used in industries as a solvent
There are three methods for manufacturing isopropyl alcohol
The term rubbing alcohol was coined in the US in the 1920s
What is Isopropyl Alcohol?
Isopropyl alcohol is a common compound in the alcohol family, a multi-purpose chemical which is widely-used as a solvent because of its ability to dissolve oils and resins, amongst other things. It is a colourless, flammable, volatile liquid which has a strong, instantly-recognisable smell.
IPA, as isopropyl alcohol is commonly known, is produced using three methods: indirect hydration of propylene, direct hydration of propylene, and catalytic hydrogenation of acetone.

Uses of Isopropyl Alcohol
Aside from being a common solvent, isopropyl alcohol has many uses such as:
- Cleaning agent
- Astringent
- Dilution and extraction in lab chemicals
- Cold-cleaning in electroplating
- Stripping paint
- Combatting muscle and joint aches
- Disinfectant
Understanding Rubbing Alcohol
‘Rubbing alcohol’ is a term coined in the 1920s prohibition era in the US, when it was used as a massage liniment rubbed on the skin.
There are two common types of rubbing alcohol: isopropyl alcohol (isopropanol) and ethyl alcohol (ethanol).
Rubbing alcohol products are commonly sold in 70% concentrations, which is the optimal concentration for killing germs.
In some countries, rubbing alcohol products are also sold in 40% concentrations, which is mild enough not to dry or irritate the skin, but strong enough as a cleaning agent.
Perfume is also typically added to rubbing alcohol products to mask the pungent odour of the alcohol.
What Does Isopropyl Alcohol Have To Do With Rubbing Alcohol?
Rubbing alcohol is either isopropyl alcohol or ethyl alcohol that has been mixed with water. In Britain it’s also known as surgical spirit, according to the British Pharmacopoeia. Rubbing alcohol which has been made from isopropyl alcohol is the most widely available, and is used mainly as an antiseptic in the home. It is for external use only.
Manufacturers of rubbing alcohol can make the solution according to their own formulation and standards, with a range of alcohol content between 70-99% v/v. The most common formula contains 70% isopropyl alcohol, the remainder being water, denaturants, and perfume oils.
Rubbing alcohol is definitely not the type of alcohol you would drink, because of its high proof. Isopropyl-based rubbing alcohol doesn’t contain the ethyl alcohol that you need in alcoholic drinks; and ethyl-based rubbing alcohol uses denatured alcohol, which includes at least one bitter poison that renders it toxic.
Why Is It Called Rubbing Alcohol?
The name ‘rubbing alcohol’ was coined in the United States during the 1920s, for two reasons. Firstly, the solution was used as a liniment in massages, and was literally rubbed in. During this time different additives and perfumes were added, so it didn’t have quite the pungent aroma it has today.
Secondly, this was Prohibition-era and there was a need to distinguish rubbing alcohol from alcoholic beverages. Despite that, there were instances of rubbing alcohol being used as a drink – with ill effects.

Other Uses of Rubbing Alcohol
Rubbing alcohol can also be used as a household cleaner when mixed with water. Make sure you use it in a well-ventilated area away from heat sources, as both the fumes and solution are flammable.
This mixture is great for cleaning stainless steel, mirrors, and glass as it dries streak-free. You can also use it to remove permanent marker and even defrost your car windows.
Key Differences Between Isopropyl Alcohol & Rubbing Alcohol
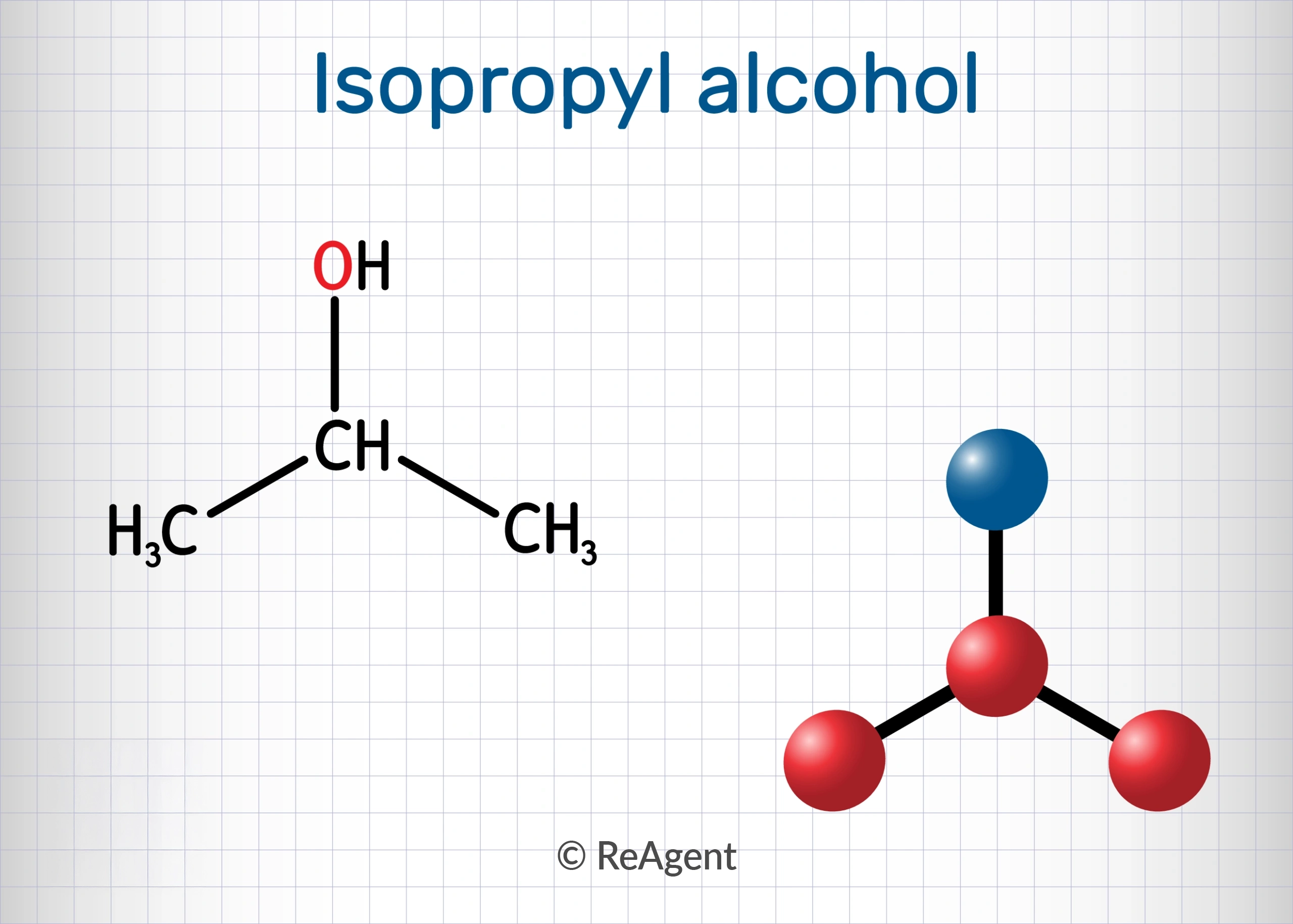
Isopropyl alcohol is a specific type of alcohol with the chemical formula C3H<O, also written as CH3CHOHCH3.
Its structural formula is shown below. Isopropyl alcohol is one of the two types of alcohol that are sold as rubbing alcohol, the other being ethyl alcohol. Rubbing alcohol is typically sold at 70% concentration and is used as an antiseptic, a liniment, and a cleaning agent.
Composition and Purity
Rubbing alcohol is typically composed of either isopropyl or ethyl alcohol.
The most common concentration is 70%, but in some countries it is also sold in 40% concentrations.
Aside from alcohol and water, perfume oils and denaturants such as denatonium benzoate are also added.
Effectiveness and Applications
Rubbing alcohol is applied on the skin or on and around a wound and is used as a skin cleaning agent and as antiseptic. At 70% concentrations, it can kill a wide range of germs, which include bacteria, fungi, and even some viruses. In fact, it can typically kill about 60 to 90% of germs.
Safety and Handling
Rubbing alcohol is highly flammable and toxic. You need to keep it out of reach of children.
It must also be stored in a cool place and you should always keep the cap on your container. Never put it near a fire or any potential ignition source, and keep your original container.
Conclusion
Isopropyl alcohol is used as a primary active ingredient of rubbing alcohol. However, not all rubbing alcohol products contain isopropyl alcohol. Some instead contain ethyl alcohol as the primary ingredient. These products are commonly sold at 70% concentrations and they are used as antiseptic. Isopropyl alcohol also has several industrial applications, mainly as solvent.
At ReAgent, we manufacture isopropyl alcohol and rubbing alcohol in a range of sizes. Contact our expert team today to discuss your requirements.