Isomerism is the molecular property of substances that have the same chemical formulas but different molecular arrangements.
The molecular structures are almost identical, but in reverse. Isomers are mirror images of each other that cannot be superimposed because some of the elements are located in reversed spatial locations. This property sometimes leads to different chemical behaviours.
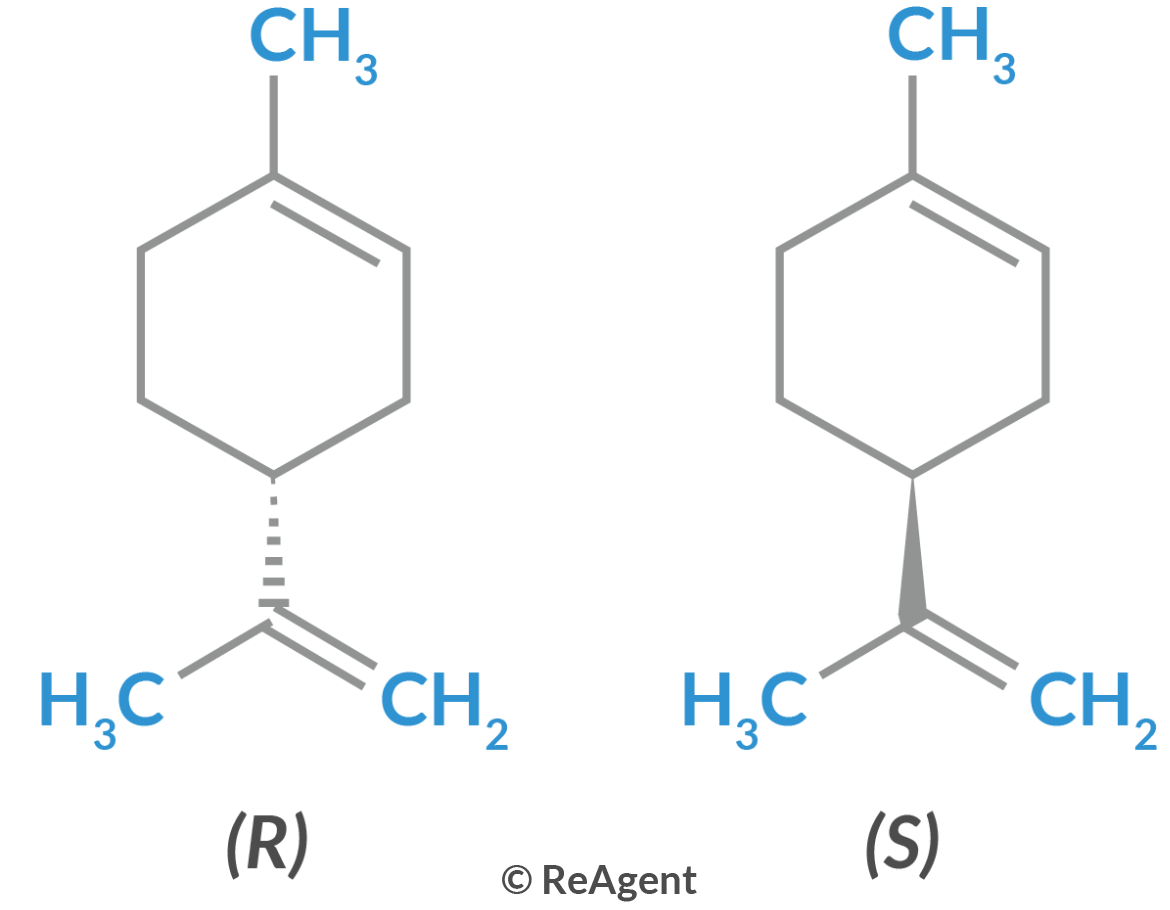
Specifically, optical isomerism is a type of isomerism that occurs in organic compounds containing an asymmetric carbon atom. This carbon forms stereoisomers that have opposite effects on plane polarised light. Optical isomerism is possible only found in molecules with a single chiral centre. A chiral centre is an atom with tetrahedral structure in a molecule that has four different ligands. Common examples of atoms that serve as chiral centres are carbon, sulphur, and nitrogen.
In this post:
Molecular Structures of Chemicals
The unique identity of substances are mainly distinguished by their chemical compositions. Their physical properties and reactivity are largely determined by the collective atoms that are bonded together into molecular units. In turn, these molecular units are arranged in certain ways that determine the macroscopic structure of a substance, such as the crystalline structures of most salts. However, some chemicals have isomers.
Optical isomerism occurs both in inorganic and organic chemicals. These isomers, also called enantiomers, typically have the same chemical properties and very similar physical properties. However, many of these enantiomers act very differently in biological systems. For example, the scent molecule, limonene, has two isomers. One isomer gives the distinctive scent of oranges while the other isomer smells like pine.
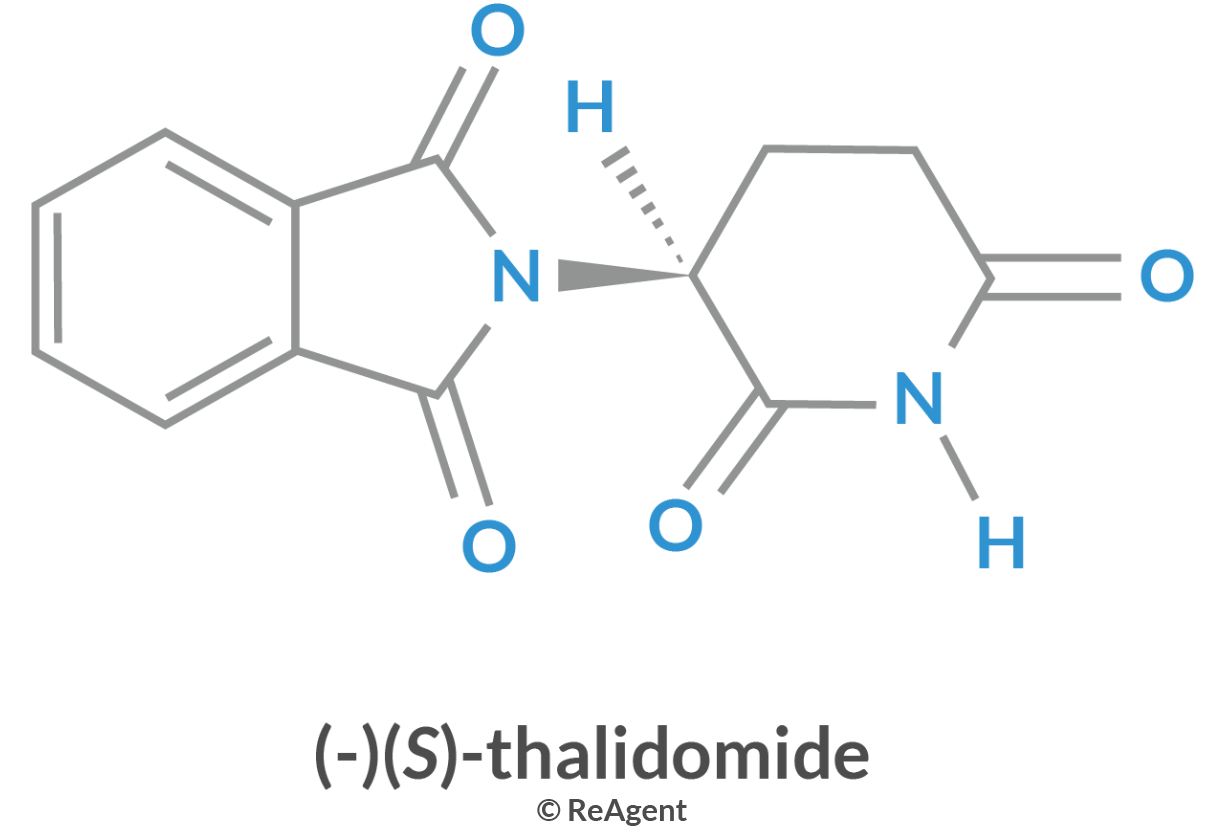
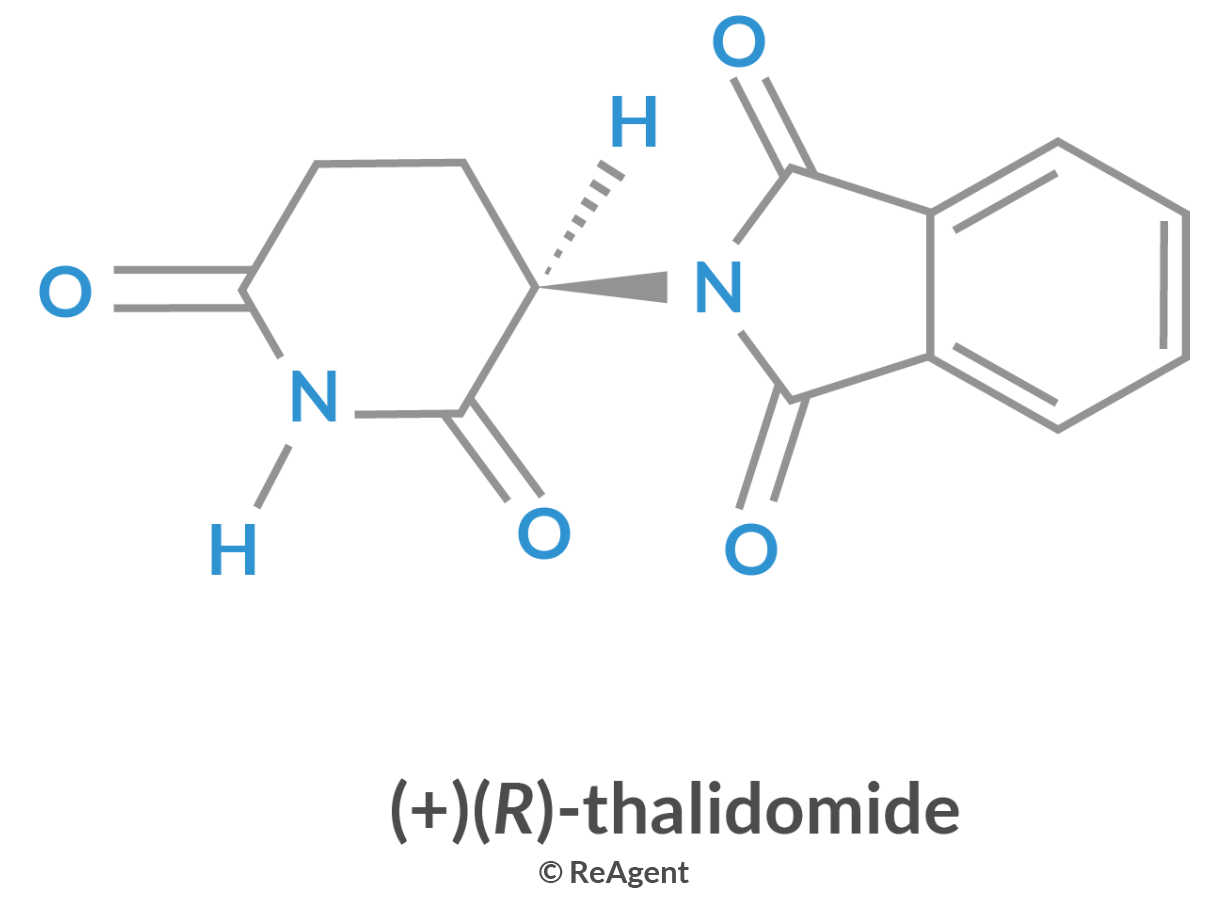
Another example of the drastic effect of isomerism in biological systems is the birth defects caused by thalidomide isomers. Thalidomide was an anti-nausea prescription drug given to pregnant women in the 1950s. One of the isomers of the drug caused serious birth defects. When the detrimental effect of the drug was definitively proven, it was immediately removed from the market in 1961.
Chirality and Stereoisomers
Molecules are either chiral or achiral. The first type of molecules do not have any plane of symmetry. Meanwhile, the latter type of molecules have one or more planes of symmetry. A chiral molecule cannot be superimposed on its mirror image. An achiral molecule is superimposable.
Optical isomers are stereoisomers because they vary in terms of the spatial arrangements of their component atoms. However, they have the same atomic composition and connectivity. This gives rise to the concept of right-handedness or left-handedness of a molecule with optical isomerism.
This affects the way these molecules rotate polarised light. If the rotation is clockwise (dextrorotatory, symbolised by the plus sign), the molecule is right-handed. Conversely, if the rotation is counterclockwise (levorotatory, symbolised by the minus sign) the molecule is left-handed.
If the sample mixture of isomers has a 50:50 ratio of opposite isomers, it’s called a racemic mixture. This type of mixture is optically inactive, which means that polarised light is not rotated at all, whether clockwise or counterclockwise. Therefore, the purity of an isomer mixture can be calculated based on how much polarised light is rotated and in which direction.
A Special Case of Isomerism
Optical isomerism is a special case of isomerism because it affects the way polarised light is rotated as it passes through the isomer. In most cases, organic isomers are liquid and transparent or translucent when dissolved in a solution. Therefore, they can affect the rotation of polarised light waves.
Optical isomers can be complex and large molecules but they must have the following characteristics:
- One chiral carbon centre: Organic molecules like amino acids can have more than one chiral carbon centre, but they will not exhibit optical isomerism if there is more than one chiral centre. Except for glycine, all amino acids have at least one chiral centre.
- Tetrahedral atom as the centre: This means that there must be four valence pairs of electrons that allow the atom to have a tetrahedral shape.
- Four different types of ligands: This means that four atoms, functional groups, or molecules must be attached to each valence pair of electrons. This results in the asymmetry of the optical isomers.
Below is a diagram that illustrates how plane polarised light is rotated by mirror-image isomers (optical isomers):  Optical isomers are types of geometric isomers but differ only in one aspect: the way they interact with polarised light. Other geometric isomers have a wide variety of arrangements in terms of single atoms, bonds, and rings. However, they may not necessarily exhibit the characteristics of optical isomers.
Optical isomers are types of geometric isomers but differ only in one aspect: the way they interact with polarised light. Other geometric isomers have a wide variety of arrangements in terms of single atoms, bonds, and rings. However, they may not necessarily exhibit the characteristics of optical isomers.
Many geometric isomers also have widely different chemical and physical properties. For example, cis- and trans-2-butene have very different boiling points and densities. Cis-2-butene has a boiling point of 4°C and density of 0.616 g/cu cm at 25 °C. Meanwhile, trans-2-butene has a boiling point of 2.25 °C and a density of 0.6042 g/cu cm at 20.4 °C.
For more revision resources, visit our A level chemistry hub.