If you’re revising energetics for your upcoming chemistry A level exam, it’s important to reinforce your knowledge of physics and maths. You’ll encounter a lot of questions in your A level chemistry exam that will require you to compute the energy involved in chemical reactions, so understanding the right units of measurement, conversions, constants, and equation manipulations is crucial.
Equally important is being able to analyse given problems in terms of the energy of reaction and the rate of reaction. You must also be able to identify whether the reaction occurred in an open, closed, or isolated system.
Luckily, most of the problems you’ll be asked to solve in your chemistry exam will be based on actual experiments, many of which you’ve probably performed in class. This will give you an advantage because although the exact details of the problem will vary, the underlying principles will remain the same – so as long as you know these, you’re set!
In this post:
System and Surroundings
The first concept you should understand when it comes to analysing the energetics of a reaction, whether chemical or physical, is the relationship between a system and its surroundings. Knowing the type of system where chemical or physical reactions occur is essential in calculating the energetics of the process.
A system, which includes a substance or substances being studied, can either be an open, closed, or isolated system. In terms of chemical reactions, a system refers to the atoms of the reactants and their bonds. The surroundings are everything else, such as the container, the non-participating solvent, and the air.
- Open system: This refers to a system that allows the exchange of matter and energy with the surroundings. An example of this is a gasoline combustion engine, which releases energy in the form of heat and mechanical energy. It also releases the byproducts of combustion as smoke (in the case of incomplete combustion) or as steam and carbon dioxide.
- Closed system: A closed system allows the exchange of energy with its surroundings – but not the exchange of matter. One good example of this is David Latimer’s terrarium, which he sealed in 1960. Since then, it has become a sustaining micro-ecosystem.
- Isolated system: An isolated system does not allow the exchange of energy or matter with its surroundings under certain conditions. In reality, there’s no such thing as a perfect isolated system. Perhaps the only true isolated system is the universe itself. However, for practical purposes, many isolated systems occur in nature as well as in the artificial environment of a chemistry lab. For example, a hot coffee sealed inside a thermos flask is an isolated system to some extent. Nonetheless, the heat will inevitably leak and dissipate outside due to conduction and radiation, albeit very slowly.

Kinetics and Thermodynamics
While kinetics and thermodynamics are related, they’re also distinct concepts:
- Kinetics is all about the rate of reaction and the intermediate steps between the initial and final state of the reaction.
- Thermodynamics is concerned with the energy difference between the initial and final state of the reaction.
In the case of neutralisation reactions between acids and bases, the kinetics is determined by the strength of the acids and bases under standard conditions, which include specific concentrations. The strength of an acid or base is usually measured in terms of pH and dissociation constants.
You can measure the energy involved in a chemical reaction using a number of energy units, such as Joules or calories. To measure the energy, you must first determine whether the reaction is exothermic or endothermic. Exothermic reactions release heat energy to the surroundings, while endothermic reactions absorb heat energy from the surroundings.
Below is a diagram of an endothermic reaction. As you can see, the products have higher energy than the reactants:
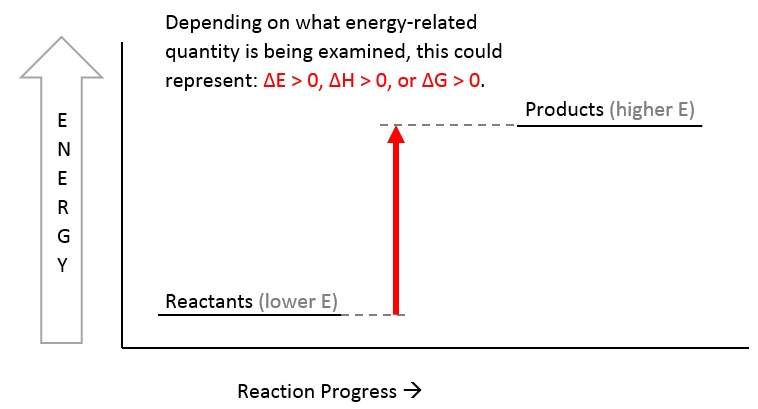 Meanwhile, an exothermic reaction has reactants that have high energy initially, but release this to the surroundings over time, as shown in the diagram below. Therefore, the final products have lower energy compared to the reactants:
Meanwhile, an exothermic reaction has reactants that have high energy initially, but release this to the surroundings over time, as shown in the diagram below. Therefore, the final products have lower energy compared to the reactants:
 Chemical Energy
Chemical Energy
In chemistry, energetics is focused on the potential and kinetic energy of chemicals. All chemicals have potential energy. This energy is stored in the bonds between the atoms, unlike nuclear energy, which is stored in the nucleus. When substances react, they break and make new bonds. In the process, energy is either released (exothermic) or absorbed (endothermic).
In physics, the definition of energy is its capacity to do work. The SI unit of energy is Joule, which is defined as one Newton of force applied to one kilogram of mass moved by one metre. Therefore, the Joule unit is expressed as kg⋅m2⋅s−2. It’s also equal to 1W⋅s or 2.390×10−4 kcal (thermochemical calorie).
The physics formulas for energy are as follows:
 Although these indicate mass, velocity, and gravitational constant, they’re useful in calculating chemical energy through mechanical conversions. For example, the chemical energy of gunpowder can be calculated based on the kinetic energy of the bullet.
Although these indicate mass, velocity, and gravitational constant, they’re useful in calculating chemical energy through mechanical conversions. For example, the chemical energy of gunpowder can be calculated based on the kinetic energy of the bullet.
If you want to be more precise in calculating the change in energy involved in a chemical reaction (whether endothermic or exothermic), you can simply subtract the energy taken out from the energy taken in. You can either measure this in terms of heat, or use the bond energy. Each covalently bonded molecule has a bond energy, which is experimentally determined and expressed in kiloJoule per mole of a substance. Bond energy is the heat energy or enthalpy required to break a mole of molecules into its constituent atoms.
Take this example of the reaction between hydrogen and chlorine to form hydrogen chloride:
H2 + Cl2 → 2HCl
The corresponding bond energies are as follow:
- Energy in = 436 + 243 = 679 KJ / Mole
- Energy out = 2 x 432 = 864 KJ / Mole
- Energy change = 679 – 864 = -185 KJ / Mole
Based on the energy change, we can see that it’s an exothermic reaction.
What is Enthalpy?
All chemical reactions involve an energy change, which can either be positive or negative. Exothermic reactions, like the example above, have a negative energy change because the reactants release heat when they react. Conversely, the energy change in endothermic reactions is positive because the reactants absorb energy during the reaction.
These reactions can be illustrated by energy diagrams, like the generalised energy diagrams below:
The enthalpy of a system is the total internal energy plus the product of the pressure and volume of a thermodynamic system. Therefore, it can be expressed as:
H = E + PV
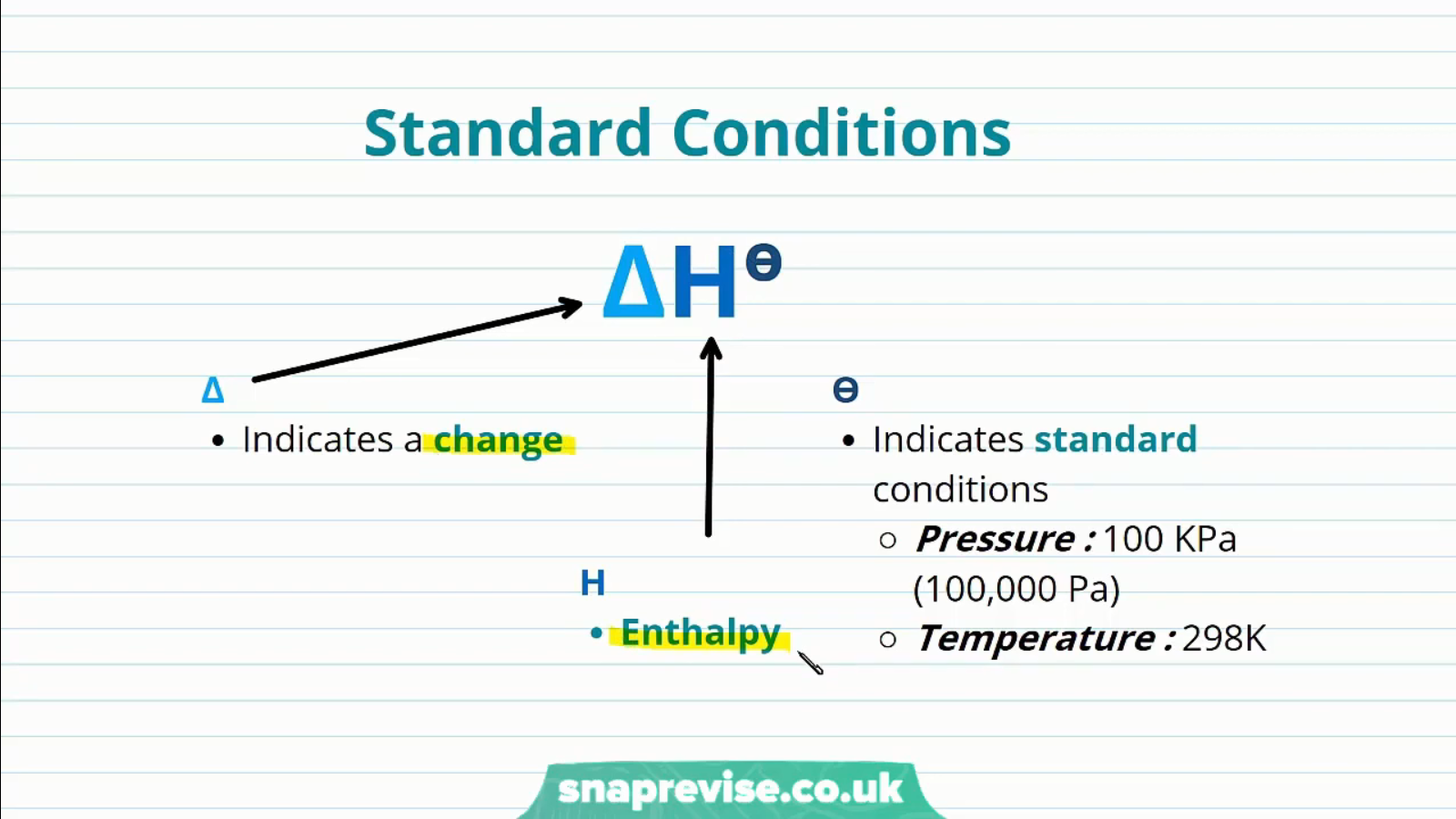
The enthalpy of a system is measured under standard conditions, which are illustrated below. All chemical reactions involve change in the enthalpy of a thermodynamics system:
Chemical reaction enthalpies can be classified into different categories, but under the standard conditions:
- Enthalpy change of formation: The change in energy involved when a mole of a substance is formed from its constituents
- Enthalpy change of combustion: This is the change in energy that occurs when one mole of a substance is completely combusted
- Enthalpy change of neutralisation: This is the change in energy associated with the formation of one mole of water from the reactions of acids and bases under standard conditions
Hess’s Law
Hess’s Law is named in honour of the Swiss-born Russian chemist and medical doctor, Germain Hess, who proposed the concept. His law governs enthalpy, stating that:
“The change of enthalpy in a chemical reaction is independent of the pathway between the initial and final states.”
Simply put, the heat or energy involved in a chemical reaction under standard conditions are not affected by the route of chemical reactions between the initial and final states. It’s also a principle implied by the first law of thermodynamics or the conservation of energy. This means that whether or not there are intermediate products of reactions, the change in enthalpy is not affected.
For more A level chemistry resources to help you revise and for information on studying chemistry A level, check out our A Level Chemistry Resources hub.