Titanium dioxide is a simple oxide of titanium that’s extracted from naturally occurring minerals, namely ilmenite, rutile, and anatase. When purified from its natural mineral forms, titanium dioxide is powdery white. It’s mainly used as a pigment in paint, and is also a common ingredient in ink, sunscreen, and food colouring.
In this post:
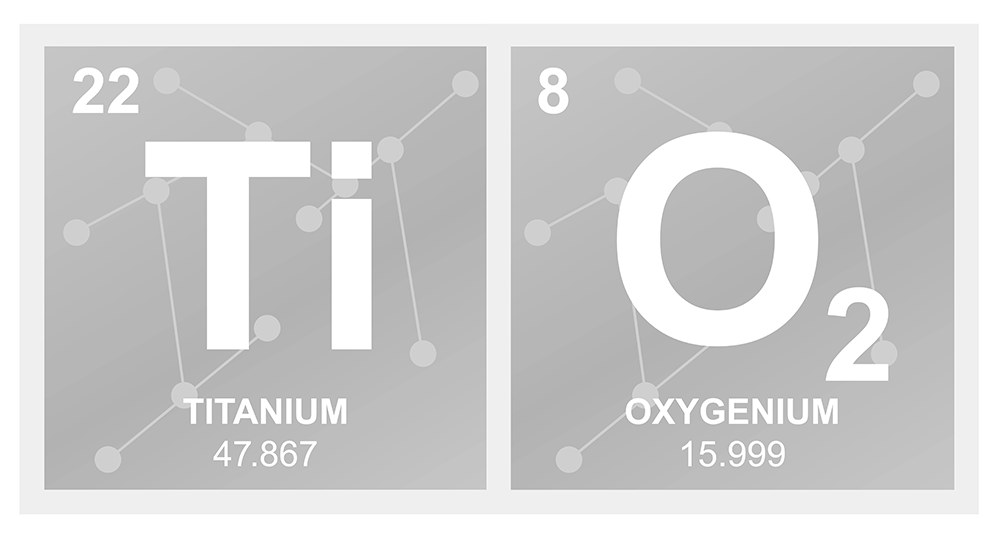
What Is The Chemical Formula For Titanium Dioxide?
Titanium dioxide has the chemical formula TiO2.
It doesn’t actually naturally occur in pure form. Instead, it’s usually found in combination with other compounds, such as igneous rocks. For instance, in rutile form, it’s commonly found in quartz crystals.
Naturally occurring rutile minerals may contain up to 10% iron as well as significant amounts of niobium and tantalum. Rutile is the most common source of titanium dioxide in nature. Rarer polymorphs of the compound are anatase, akaogiite, and brookite.
Polymorphs have the same chemical formula but different crystalline structures. For example, rutile has a tetragonal crystalline form, while brookite has an orthorhombic structure. Orthorhombic is a crystalline structure with three unequal axes at right angles. Meanwhile, tetragonal forms have crystal lattices that are formed from stretching a cubic lattice along one of its lattice vectors. This arrangement makes the cube a rectangular prism with both the base and height as squares.
What Are The Applications Of Titanium Dioxide?
The economic demand for titanium dioxide is mainly based on its properties as a pigment and UV blocker. It’s extracted and processed as an ingredient for various products, mainly related to the following applications:
- Pigment: TiO2 was first mass-produced in 1916 to make pigments. Given its white colour, it’s useful for making products opaque, and is used in paints, coatings, plastics, papers, inks, foods, and even medicines. As a paint pigment, it produces a perfect white because of the crystalline structure of the titanium dioxide particles.
- Sunscreen: TiO2 is used as an ingredient in sunscreen because it can block the sun’s ultraviolet rays, with UV radiation bouncing off its particles.
- Thin reflective coating: The high reflective index of TiO2 makes it an excellent refractive optical coating when deposited as a thin film, for example on dielectric mirrors and optics.
- Cosmetics: The high refractive index of TiO2 crystals also provides a shiny look in makeup and lipsticks. TiO2 is also used to add a white tint to powders and highlighters, while also adding a small level of sun protection.
- Ceramic glazes: The crystalline structure of TiO2 is responsible for its high refractive index and white shiny colour. This makes it ideal for ceramic glazes.
Find out more about the applications of titanium dioxide in our blog post: What is titanium dioxide used for?

Is Titanium Dioxide Safe?
The International Agency for Research on Cancer has flagged titanium dioxide as possibly carcinogenic to humans. As a result, some countries have started considering a ban on the use of the compound in many products, particularly in food colouring, cosmetics, and toothpastes.
However, it’s crucial to point out that the conclusions of IARC are based on highly specific evidence relating to animal models. Experimental rats that were exposed to pigment-grade particles of the compound resulted in the rats developing respiratory cancer. While this shows that there is definitely potential for TiO2 to act as a carcinogen, there have been no conclusive meta studies on humans as yet.
Is Titanium Dioxide Harmful?
Titanium dioxide is generally safe in small quantities and when not ingested or inhaled. Paints and other substances that are solid when dried pose very low risks. Long-term studies show that there are no occupation-related hazards associated with the substance, either.
One of the greater hazards of TiO2 is thought to be when the particles are nano size. Food and other products like cosmetics contain small amounts of nano-size TiO2 particles. In this state, the particles tend to bind together to form larger-size particles, giving them a chance to accumulate. However, the European Food Safety Authority (EFSA) has declared titanium dioxide safe when used as a food additive.
How Is Titanium Dioxide Manufactured?
Titanium dioxide was first mass-produced as a pigment in 1916. The most common mineral source is ilmenite. Rutile mineral sand can also be processed to produce the pure form of the compound. The most common manufacturing method used, however, is the chloride process, which is used to separate titanium from its ores:
TiO2 + C → Ti + CO2
Ti + 2Cl2 → TiCl4
TiCl4 + O2 → TiO2 + 2Cl2
The sulfate process is also used by some processing plants to produce the pigment in crystal form. The same process can also extract the compound and produce the anatase form of titanium dioxide. Anatase, a metastable mineral form of TiO2, is commonly used in paper to make the colour whiter.
Several steps are needed for the sulfate process of manufacturing titanium dioxide:
- The titanium ore, usually ilmenite, is dissolved in sulfuric acid to form titanyl sulfate (TiOSO4)
- Titanyl sulfate then undergoes hydrolysis, resulting in an insoluble and hydrated from of TiO2
- The solid TiO2 is heated in a calciner to evaporate any water and decompose any sulphuric acid
- Once it’s cooled, the solid product is then formed into white crystals
Processing plants using the sulfate process for manufacturing TiO2 need concentrates of ilmenite minerals. If these are unavailable, other suitable sources of titanium can be pretreated. Iron is first extracted from ilmenite by treating it with sulphuric acid. This produces rutile mineral, which is further processed based on the intended use such as pigment grade substance.
Ilmenite can also be processed using the Becher process. This involves oxidising the mineral in order to separate the iron component. Ilmenite as well, as other sources of titanium, can be treated with elemental chlorine to produce tetrachloride. Oxygen is then introduced to regenerate the chlorine and finally form the titanium dioxide compound.
The estimated global production of TiO2 is primarily fuelled by the demand for white pigment. Global production of pigment-grade titanium dioxide is estimated to be an average of 5.3 metric tonnes per year. In 2014, the world production of the compound in its various grades and applications exceeded nine billion tonnes.
Is Titanium Dioxide A Metal?
Although titanium dioxide contains a metal, it is not a metal. It’s actually a type of mineral with various forms, occurring naturally in igneous and metamorphic rocks along with other minerals.
The main thing that gives away that this substance isn’t a metal is that it doesn’t have the common characteristics of other metals, like electrical conductivity or malleability. Additionally, metals are always in elemental form, while titanium dioxide doesn’t naturally occur as an element; it has to be extracted from minerals like ilmenite and rutile.











