Although both USP (United States Pharmacopoeia) and EP water (European Pharmacopoeia) must meet strict quality standards, there are some important differences between them.
USP purified water is commonly used to manufacture sterile medical products such as parenteral items, surgical dressings, sutures, ligatures, and ophthalmic preparations.
EP purified water, on the other hand, is typically used to produce non-sterile medical or pharmaceutical products. As these items don’t necessarily require zero microbial contamination, a higher conductivity threshold is acceptable.
Continue reading to find out more about the differences between USP and EP water and how to decide which one is most suitable for your needs.
In this post:
Choosing between USP and EP water
Deciding whether to use USP or EP water largely depends on the type of products you’re planning to manufacture or the experiment you’re going to perform.
If you intend to produce sterile products for the medical or pharmaceutical sector, it’s best to choose USP grade water. This is because it contains very few impurities and has a low conductivity limit.
Sterile products are used in various surgical and ophthalmic procedures. They also include things like injections, which deliver medicines internally. These types of products must be completely sterile to prevent infections and potential complications like sepsis.

In some instances, however, medical products don’t have to be absolutely sterile. This is typically the case for non-parenteral medications that are delivered orally or topically, as opposed to puncturing the skin. For these types of products, EP grade purified water is usually suitable.
About USP water
What is USP water?
USP is an acronym for United States Pharmacopoeia, a set of standards used by US-based pharmaceutical companies when manufacturing certain products.

USP water is a type of highly purified water that meets those standards. It’s commonly used in the manufacture of sterile pharmaceutical products.
What is the specification of USP water?
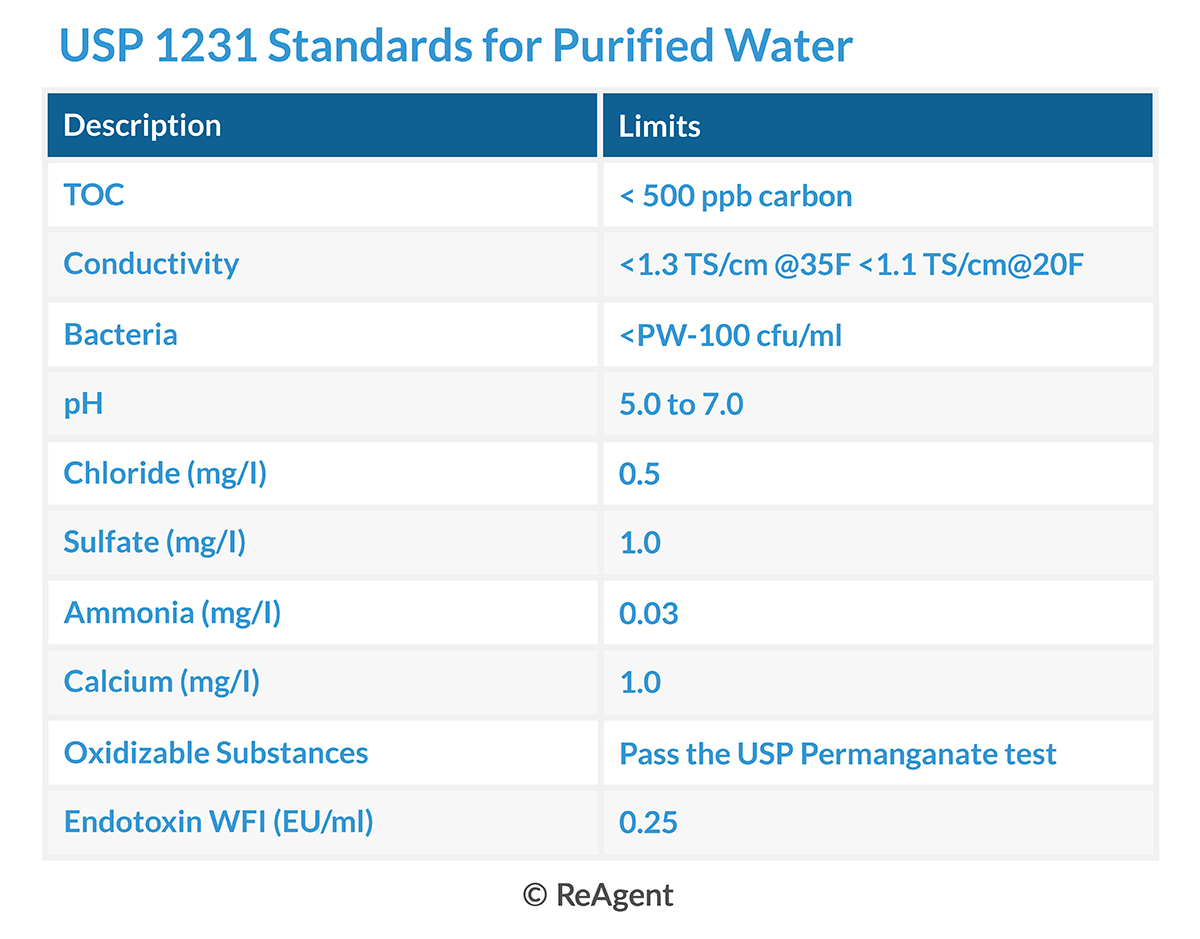
USP purified water must meet strict specifications and thresholds. These include parameters on the total organic carbons (TOC), as well as conductivity, bacteria, pH, chlorine, sulphate, ammonia, calcium, oxidisable substances, and endotoxin. See the table below for more information.
All of the USP water we manufacture here at ReAgent Chemicals meets these stringent requirements. We also test the water regularly to ensure it continues to comply with the strict parameters set out by the United States Pharmacopeia.
About EP water
What is EP water?
EP stands for European Pharmacopoeia, an internationally recognised set of standards for pharmaceutical companies in Europe.
EP water refers to a type of purified water that’s used in the manufacture of non-sterile medical and pharmaceutical products.
What is the specification of EP water?
The specifications for EP water include various parameters on total organic carbons, nitrates, chlorides, microbial limits, and oxidisable substances.
There are acceptable limits based on the thresholds set out by the European Pharmacopoeia. Refer to the table below for more details.
Regulations of USP and EP water
USP and EP purified water are governed by stringent regulations and best industry practices based on international, national and regional standards. There are also corresponding standards set by juridical legislation, as well as various treaties and trade, safety and environmental laws.
For example, the Occupational Safety and Health Act of 1970 is a federal law enforceable in the United States. It applies to both the private and government sectors.
The framework of the European Pharmacopoeia itself is made mandatory by the following laws:
- The Convention developed by the Council of Europe on the Elaboration of a European Pharmacopoeia.
- A Protocol adopted in 1994 and amending the Convention and defining the respective powers of the EU and its member states within the European Pharmacopoeia Commission.
- The European Union Directive 2001/83/EC as amended. These maintain the mandatory character of European Pharmacopoeia monographs when requesting marketing authorisation.
Conclusion
Although there are similarities between the standards for USP and EP purified water, the applications of these two products differ. USP purified water is used in the manufacture and preparation of sterile medical products, while EP purified water is used for non-sterile medical products.
Various national, regional, and international treaties, laws, and industry standards regulate USP and EP purified water production. These are based on specific parameters and corresponding thresholds.We sell a wide variety of high-quality water products, including both USP and EP purified water. You can view our full range in our online shop.