Period 3 elements are those in the third row of the periodic table. The way period 3 elements form oxides, and how they react with other chemicals, is related to their positions in the periodic table. Furthermore, you can compare the relative atomic radius, ionisation energy, and electron affinity of the eight period 3 elements. The period 3 elements are:
- Sodium
- Magnesium
- Aluminium
- Silicon
- Phosphorous
- Sulphur
- Chlorine
- Argon
What Are the Properties of Period 3 Elements?
While the eight period 3 elements have some similarities, they also have many differences – or rather, increasing differences. For example, aluminium has lower ionisation energy, and a larger atomic radius, compared to silicon.
The rows of the periodic table refer to periodicity, which is related to the various chemical and physical properties of the elements, either in increasing or decreasing order. For instance, given their position in the row, we immediately know that sodium is more reactive than magnesium, and indeed any other element in period 3. 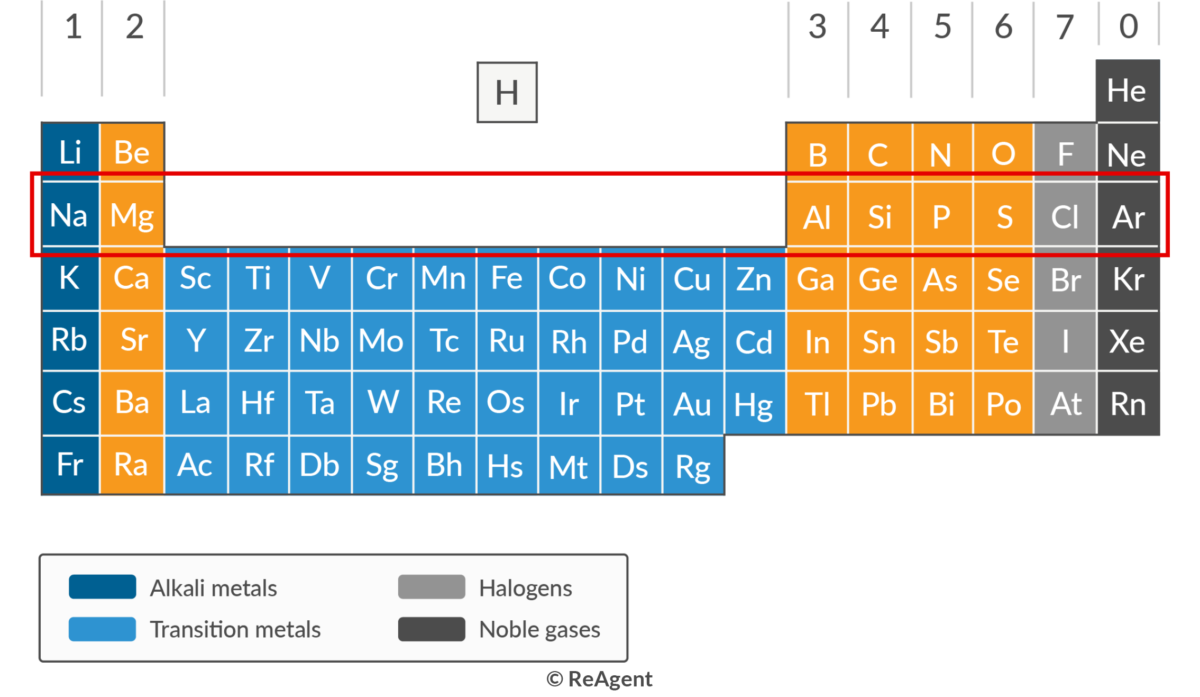
All the period 3 elements have their first three electron shells filled. This corresponds to the neon (Ne) electron configuration, which is [Ne]=1s2 2s2 2p6. As a result, the nuclear charge is shielded from the valence electrons in the outer energy level.
Some of the the overall trends for period 3 elements as you move from left to right are the following:
- Each of the eight elements of the period belong to a group, jumping from Groups 1 (alkali metals) and 2 (alkaline earth metals) to Groups 13 (boron group), 14 (carbon group), 15 (pnictogens), 16 (chalcogens.), 17 (halogens), and 18 (noble gases). The transition metals belong to period 4, just below period 3.
- The outermost shells are successively filled with electrons in this manner: first the 3s orbitals (3s1 and 3s2), then the 3p orbitals (3p1 to 3p6).
- With small dips in Group 3 and Group 6, the first ionisation energy of the period 3 elements increases from left to right.
- As you move from left to right, the maximum oxidation increases based on the number of valence electrons of the element.
- Elements in group 3 start with metallic elements (sodium, magnesium, aluminium), then metalloid or semiconductors (silicon), and finally non-metallics (phosphorus, sulphur, chlorine, argon). As a result, the conductivity decreases from left to right. For example, sodium has a conductivity of 21 × 106 Siemens per meter (S/m) while argon has zero conductivity.
- The bonding types increasingly become stronger, from covalent to ionic bonds. This applies to oxide, halide, and hydride compounds of period 3.
- The atomic radii has a steady decrease from left to right as the nucleus becomes more crowded. The main reason is the stronger attraction force of the nucleus.
- Melting points tend to increase from left to right until we reach silicon, then they start to decrease. The graph and table below show this trend. As you can see, there is a significant dip after silicon before it slightly increases and then drops again.

What Are the Chemical Reaction Trends of Period 3 Elements?
The chemical reaction trends of the eight period 3 elements can be categorised into two types: reaction with water and reaction with oxygen. Furthermore, we can also see trends in terms of the reactions of the oxides of these elements with water. In the presence of water, the oxides can either form acidic or basic solutions, or new substances altogether.
The general trend is that reactivity decreases from left to right, with the exception of chlorine. By this logic, sodium, which is positioned to the far left, is highly reactive with both water and oxygen, but argon, the rightmost element in the row, is a completely inert gas. This is mainly to do with the electron configuration of the elements. When there’s a lower number of valence electrons to lose, it translates to higher reactivity.
Reactions with water
Only sodium reacts with water at room temperature. The rest of the period 3 elements are unreactive with pure water at room temperature and at normal one atmosphere pressure.
Sodium violently reacts with water to form a strong basic substance, sodium hydroxide:
2Na + 2H2O → 2NaOH + H2
Magnesium only reacts vigorously with water at high temperatures. For example, it reacts with steam to form magnesium oxide. Hydrogen is also released in this reaction:
Mg + H2O → MgO + H2
Magnesium can also react with water at lower temperatures, but it’s a very slow process. The reaction forms magnesium hydroxide:
Mg + 2H2O → Mg(OH)2 + H2
Reactions with oxygen
With the exception of argon, which is a noble gas, all the period 3 elements react with oxygen at various proportions and conditions. Chlorine and sulphur can form more than one type of oxide, based on the oxidation state. Here is a list of the period 3 elements’ reactions with oxygen:
4Na + O2 → 2Na2O
2Mg + O2 → 2MgO
4Al + 3O2 → 2Al2O3
Si + O2 → SiO2
P4 + 5O2 → P4O10
S8 + O2 → 8SO2
Basic oxides
Some oxides form base solutions in water as the ions separate. The oxides of period 3 elements react with water to form new compounds. Here are some examples:
Na2O + H2O → 2NaOH
Na2O + H2SO4 → Na2SO4 + H2O
MgO + H2O → Mg(OH)2
MgO + 2HCl → MgCl2 + H2O
Acidic oxides
Some oxides form acid solutions in water as the ions separate and react with water. Here are some examples:
P4O10 + 6H2O → 4H3PO4
SO2 + H2O → H2SO3
SO3 + H2O → H2SO4
As you can see, the metallic oxides form basic compounds as they react with water. Meanwhile, the semi metallic and nonmetallic oxides form acidic solutions or compounds. Other reactions will require special conditions to occur, such as higher temperatures. In some cases, the reactants are not fully consumed.
The strength of the acid or base is not directly proportional to the concentration of pH. The more accurate measure of the acid or base strength is the dissociation constant, which refers to the extent by which the ions dissociate in water.
For more A level chemistry resources to help you revise and for information on studying chemistry A level, check out our A Level Chemistry Resources hub.












